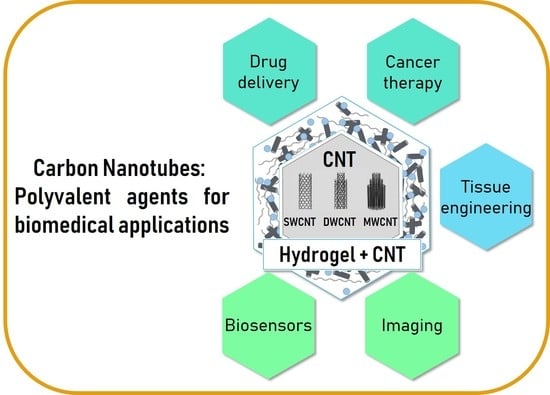
Overview of Carbon Nanotubes for Biomedical Applications
Abstract
:1. Introduction
2. Carbon Nanotubes (CNT) for Biomedical Applications
2.1. CNT Use for Diagnostic
2.1.1. Biosensors Based on CNT
2.1.2. Imaging Methods Based on CNT
2.2. CNT Use for Tissue Engineering
2.3. CNT Use for Targeted Therapies
2.3.1. CNT as Carrier for Drugs and Gene Delivery
2.3.2. Anticancer Therapies Based on CNT
3. CNT-based Hydrogels for Biomedical Applications
3.1. Biopolymer-Based Hydrogels
3.2. CNT-Based Hydrogels for Diagnostic
3.3. CNT-Based Hydrogels Use for Tissue Engineering
3.4. CNT-Based Hydrogels for Targeted Therapies
3.4.1. CNT-Based Hydrogels Use for Drug Delivery
3.4.2. CNT-Based Hydrogels Use for Skin Delivery
4. Potential Toxicity Issues Related to CNT
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Louw-Gaume, A.; Kucki, M.; Krug, H.; Kostarelos, K.; Fadeel, B.; Dawson, K.; Salvati, A.; Vazquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Neves, V.; Heister, E.; Costa, S.; Tîlmaciu, C.; Flahaut, E.; Soula, B.; Coley, H.M.; McFadden, J.; Silva, S.R.P. Design of Double-Walled Carbon Nanotubes for Biomedical Applications. Nanotechnology 2012, 23, 365102. [Google Scholar] [CrossRef] [PubMed]
- Monthioux, M.; Serp, P.; Caussat, B.; Flahaut, E.; Razafinimanana, M.; Valensi, F.; Laurent, C.; Peigney, A.; Mesguich, D.; Weibel, A.; et al. Carbon Nanotubes. In Springer Handbook of Nanotechnology; Bhushan, B., Ed.; Springer Handbooks: Berlin, Germany, 2017; pp. 193–247. [Google Scholar]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano–Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle Interaction with Plasma Proteins as It Relates to Particle Biodistribution, Biocompatibility and Therapeutic Efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Bortolamiol, T.; Lukanov, P.; Galibert, A.-M.; Soula, B.; Lonchambon, P.; Datas, L.; Flahaut, E. Double-Walled Carbon Nanotubes: Quantitative Purification Assessment, Balance between Purification and Degradation and Solution Filling as an Evidence of Opening. Carbon 2014, 78, 79–90. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef]
- Oliveira, S.F.; Bisker, G.; Bakh, N.A.; Gibbs, S.L.; Landry, M.P.; Strano, M.S. Protein Functionalized Carbon Nanomaterials for Biomedical Applications. Carbon 2015, 95, 767–779. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon Nanotubes from Synthesis to in Vivo Biomedical Applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Grady, B.P. The Use of Solution Viscosity to Characterize Single-Walled Carbon Nanotube Dispersions. Macromol. Chem. Phys. 2006, 207, 2167–2169. [Google Scholar] [CrossRef]
- Flahaut, E.; Bacsa, R.; Peigney, A.; Laurent, C. Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes. Chem. Commun. 2003, 12, 1442. [Google Scholar] [CrossRef]
- Laurent, C.; Flahaut, E.; Peigney, A. The Weight and Density of Carbon Nanotubes versus the Number of Walls and Diameter. Carbon 2010, 48, 2994–2996. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical Applications of Carbon Nanomaterials: Drug and Gene Delivery Potentials. J. Cell. Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Peng, R.; Liu, Z. Carbon Nanotubes for Biomedical Imaging: The Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Punbusayakul, N.; Talapatra, S.; Ajayan, P.M.; Surareungchai, W. Label-Free as-Grown Double Wall Carbon Nanotubes Bundles for Salmonella Typhimurium Immunoassay. Chem. Cent. J. 2013, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, I.; Barrejón, M.; Arellano, L.M.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Grafted-Double Walled Carbon Nanotubes as Electrochemical Platforms for Immobilization of Antibodies Using a Metallic-Complex Chelating Polymer: Application to the Determination of Adiponectin Cytokine in Serum. Biosens. Bioelectron. 2015, 74, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wayu, M.B.; Pannell, M.J.; Labban, N.; Case, W.S.; Pollock, J.A.; Leopold, M.C. Functionalized Carbon Nanotube Adsorption Interfaces for Electron Transfer Studies of Galactose Oxidase. Bioelectrochemistry 2019, 125, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, J.; Fam, D.; Tok, A. Horizontally Aligned Carbon Nanotube Based Biosensors for Protein Detection. Bioengineering 2016, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Mansouri Majd, S.; Salimi, A. Ultrasensitive Flexible FET-Type Aptasensor for CA 125 Cancer Marker Detection Based on Carboxylated Multiwalled Carbon Nanotubes Immobilized onto Reduced Graphene Oxide Film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Gao, Y.; Ozsoz, M.; Mulchandani, A. Electronic Detection of MicroRNA at Attomolar Level with High Specificity. Anal. Chem. 2013, 85, 8061–8064. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Tang, X.S.; Li, D. Detection of Individual Molecules and Ions by Carbon Nanotube-Based Differential Resistive Pulse Sensor. Small 2018, 14, 1800013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Ye, B. An Efficient Electrochemical Glucose Sensor Based on Porous Nickel-Based Metal Organic Framework/Carbon Nanotubes Composite (Ni-MOF/CNTs). J. Alloys Compd. 2018, 767, 651–656. [Google Scholar] [CrossRef]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H. A Route to Brightly Fluorescent Carbon Nanotubes for Near-Infrared Imaging in Mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Sherlock, S.P.; Dai, H. Deep-Tissue Anatomical Imaging of Mice Using Carbon Nanotube Fluorophores in the Second Near-Infrared Window. Proc. Natl. Acad. Sci. USA 2011, 108, 8943–8948. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Shuo, D.; Antaris, A.L.; Chen, C.; Zhang, B.; Zhao, S.; Atochin, D.N.; Huang, P.L.; Andreasson, K.I.; Kuo, C.J.; et al. Through-Skull Fluorescence Imaging of the Brain in a New Near-Infrared Window. Nat. Photonics 2014, 8, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, F.; Choi, J.H. Single-Walled Carbon Nanotubes as Optical Probes for Bio-Sensing and Imaging. J. Mater. Chem. B 2017, 5, 6511–6522. [Google Scholar] [CrossRef]
- Yudasaka, M.; Yomogida, Y.; Zhang, M.; Tanaka, T.; Nakahara, M.; Kobayashi, N.; Okamatsu-Ogura, Y.; Machida, K.; Ishihara, K.; Saeki, K.; et al. Near-Infrared Photoluminescent Carbon Nanotubes for Imaging of Brown Fat. Sci. Rep. 2017, 7, 6272. [Google Scholar] [CrossRef] [PubMed]
- De La Zerda, A.; Zavaleta, C.; Keren, S.; Vaithilingam, S.; Bodapati, S.; Liu, Z.; Levi, J.; Smith, B.R.; Ma, T.-J.; Oralkan, O.; et al. Carbon Nanotubes as Photoacoustic Molecular Imaging Agents in Living Mice. Nat. Nanotechnol. 2008, 3, 557–562. [Google Scholar] [CrossRef] [PubMed]
- De La Zerda, A.; Liu, Z.; Bodapati, S.; Teed, R.; Vaithilingam, S.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Ultrahigh Sensitivity Carbon Nanotube Agents for Photoacoustic Molecular Imaging in Living Mice. Nano Lett. 2010, 10, 2168–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Tabakman, S.; Sherlock, S.; Li, X.; Chen, Z.; Jiang, K.; Fan, S.; Dai, H. Multiplexed Five-Color Molecular Imaging of Cancer Cells and Tumor Tissues with Carbon Nanotube Raman Tags in the near-Infrared. Nano Res. 2010, 3, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Gaufrès, E.; Marcet, S.; Aymong, V.; Tang, N.Y.-W.; Favron, A.; Thouin, F.; Allard, C.; Rioux, D.; Cottenye, N.; Verhaegen, M.; et al. Hyperspectral Raman Imaging Using Bragg Tunable Filters of Graphene and Other Low-Dimensional Materials: Hyperspectral Raman Imaging Using Bragg Tunable Filters of Graphene and Other Low-Dimensional Materials. J. Raman Spectrosc. 2018, 49, 174–182. [Google Scholar] [CrossRef]
- Hong, S.Y.; Tobias, G.; Al-Jamal, K.T.; Ballesteros, B.; Ali-Boucetta, H.; Lozano-Perez, S.; Nellist, P.D.; Sim, R.B.; Finucane, C.; Mather, S.J.; et al. Filled and Glycosylated Carbon Nanotubes for in Vivo Radioemitter Localization and Imaging. Nat. Mater. 2010, 9, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Servant, A.; Jacobs, I.; Bussy, C.; Fabbro, C.; da Ros, T.; Pach, E.; Ballesteros, B.; Prato, M.; Nicolay, K.; Kostarelos, K. Gadolinium-Functionalised Multi-Walled Carbon Nanotubes as a T 1 Contrast Agent for MRI Cell Labelling and Tracking. Carbon 2016, 97, 126–133. [Google Scholar] [CrossRef]
- Lovat, V.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef] [Green Version]
- Béduer, A.; Seichepine, F.; Flahaut, E.; Loubinoux, I.; Vaysse, L.; Vieu, C. Elucidation of the Role of Carbon Nanotube Patterns on the Development of Cultured Neuronal Cells. Langmuir 2012, 28, 17363–17371. [Google Scholar] [CrossRef]
- Matta-Domjan, B.; King, A.; Totti, S.; Matta, C.; Dover, G.; Martinez, P.; Zakhidov, A.; La Ragione, R.; Macedo, H.; Jurewicz, I.; et al. Biophysical Interactions between Pancreatic Cancer Cells and Pristine Carbon Nanotube Substrates: Potential Application for Pancreatic Cancer Tissue Engineering: pancreatic Cancer on Pristine CNT Substrates. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Correa-Duarte, M.A.; Wagner, N.; Rojas-Chapana, J.; Morsczeck, C.; Thie, M.; Giersig, M. Fabrication and Biocompatibility of Carbon Nanotube-Based 3D Networks as Scaffolds for Cell Seeding and Growth. Nano Lett. 2004, 4, 2233–2236. [Google Scholar] [CrossRef]
- Abarrategi, A.; Gutiérrez, M.C.; Moreno-Vicente, C.; Hortigüela, M.J.; Ramos, V.; López-Lacomba, J.L.; Ferrer, M.L.; Del Monte, F. Multiwall Carbon Nanotube Scaffolds for Tissue Engineering Purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Z.; Rider, A.E.; Ishaq, M.; Kumar, S.; Kondyurin, A.; Bilek, M.M.M.; Levchenko, I.; Ostrikov, K.K. Carbon Nanostructures for Hard Tissue Engineering. RSC Adv. 2013, 3, 11058. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, A.C.; Rakhra, K.; Sherlock, S.; Goodwin, A.; Chen, X.; Yang, Q.; Felsher, D.W.; Dai, H. Supramolecular Stacking of Doxorubicin on Carbon Nanotubes for In Vivo Cancer Therapy. Angew. Chem. 2009, 121, 7804–7808. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.; Vollin-Bringel, O.; Fiegel, V.; Harlepp, S.; Van der Schueren, B.; Bégin-Colin, S.; Bégin, D.; Mertz, D. Engineering of Mesoporous Silica Coated Carbon-Based Materials Optimized for an Ultrahigh Doxorubicin Payload and a Drug Release Activated by PH, T, and NIR-Light. Adv. Funct. Mater. 2018, 28, 1706996. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Bian, X.; Zhu, Z.; Ding, D.; Li, X.; Jia, Z.; Jiang, X.; Hu, Y. Covalently Combining Carbon Nanotubes with Anticancer Agent: Preparation and Antitumor Activity. ACS Nano 2009, 3, 2740–2750. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Liao, C.; Fang, Y.; Guo, B. Development of a Promising Drug Delivery for Formononetin: Cyclodextrin-Modified Single-Walled Carbon Nanotubes. J. Drug Deliv. Sci. Technol. 2018, 43, 461–468. [Google Scholar] [CrossRef]
- Shi Kam, N.W.; Jessop, T.C.; Wender, P.A.; Dai, H. Nanotube Molecular Transporters: Internalization of Carbon Nanotube−Protein Conjugates into Mammalian Cells. J. Am. Chem. Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.-P.; Muller, S.; et al. Cellular Uptake of Functionalized Carbon Nanotubes Is Independent of Functional Group and Cell Type. Nat. Nanotechnol. 2007, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, G.; Cherukuri, P.; Kingston, J.; Cognet, L.; Jr, R.L.; Leeuw, T.K.; Gumbiner-Russo, L.; Weisman, R.B.; Powis, G. In Vivo Therapeutic Silencing of Hypoxia-Inducible Factor 1 Alpha (HIF-1) Using Single-Walled Carbon Nanotubes Noncovalently Coated with SiRNA. Nano Res. 2009, 13. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, M.S.; Andrade, V.A.; Gomes, E.R.M.; Aguiar, C.J.; Moraes, E.R.; Soares, J.S.; Silva, E.E.; Lacerda, R.G.; Ladeira, L.O.; Jorio, A.; et al. Highly Efficient SiRNA Delivery System into Human and Murine Cells Using Single-Wall Carbon Nanotubes. Nanotechnology 2010, 21, 385101. [Google Scholar] [CrossRef] [PubMed]
- Sanz, V.; Tilmacîu, C.; Soula, B.; Flahaut, E.; Coley, H.M.; Silva, S.R.P.; McFadden, J. Chloroquine-Enhanced Gene Delivery Mediated by Carbon Nanotubes. Carbon 2011, 49, 5348–5358. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Gherardini, L.; Bardi, G.; Nunes, A.; Guo, C.; Bussy, C.; Herrero, M.A.; Bianco, A.; Prato, M.; Kostarelos, K.; et al. Functional Motor Recovery from Brain Ischemic Insult by Carbon Nanotube-Mediated SiRNA Silencing. Proc. Natl. Acad. Sci. USA 2011, 108, 10952–10957. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, A.; Scala, A.; Sortino, G.; Zagami, R.; Zhu, Y.; Sciortino, M.T.; Pennisi, R.; Pizzo, M.M.; Neri, G.; Grassi, G.; et al. Intracellular Trafficking and Therapeutic Outcome of Multiwalled Carbon Nanotubes Modified with Cyclodextrins and Polyethylenimine. Colloids Surf. B Biointerfaces 2018, 163, 55–63. [Google Scholar] [CrossRef]
- Kaboudin, B.; Saghatchi, F.; Kazemi, F.; Akbari-Birgani, S. A Novel Magnetic Carbon Nanotubes Functionalized with Pyridine Groups: Synthesis, Characterization and Their Application as an Efficient Carrier for Plasmid DNA and Aptamer. ChemistrySelect 2018, 3, 6743–6749. [Google Scholar] [CrossRef]
- Xu, L.; Feng, L.; Dong, S.; Hao, J.; Yu, Q. Carbon Nanotubes Modified by a Paramagnetic Cationic Surfactant for Migration of DNA and Proteins. Colloids Surf. Physicochem. Eng. Asp. 2018, 559, 201–208. [Google Scholar] [CrossRef]
- Kong, F.; Liu, F.; Li, W.; Guo, X.; Wang, Z.; Zhang, H.; Li, Q.; Luo, L.; Du, Y.; Jin, Y.; et al. Smart Carbon Nanotubes with Laser-Controlled Behavior in Gene Delivery and Therapy through a Non-Digestive Trafficking Pathway. Small 2016, 12, 6753–6766. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Ding, X.; Singh, R.; Kraft, R.A.; Levi-Polyachenko, N.; Rylander, M.N.; Szot, C.; Buchanan, C.; Whitney, J.; Fisher, J.; et al. Long-Term Survival Following a Single Treatment of Kidney Tumors with Multiwalled Carbon Nanotubes and near-Infrared Radiation. Proc. Natl. Acad. Sci. USA 2009, 106, 12897–12902. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, L.; Liang, C.; Xiang, J.; Peng, R.; Liu, Z. Immunological Responses Triggered by Photothermal Therapy with Carbon Nanotubes in Combination with Anti-CTLA-4 Therapy to Inhibit Cancer Metastasis. Adv. Mater. 2014, 26, 8154–8162. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, Q.; Chen, J.; Gao, H.; Fu, D.; Shen, S. Biocompatible Polydopamine-Encapsulated Gadolinium-Loaded Carbon Nanotubes for MRI and Color Mapping Guided Photothermal Dissection of Tumor Metastasis. Carbon 2017, 112, 53–62. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Q.; Liu, Y.; Ma, T.; Su, L.; Liu, S.; Shi, X.; Han, D.; Liang, F. Decorating Gold Nanostars with Multiwalled Carbon Nanotubes for Photothermal Therapy. R. Soc. Open Sci. 2018, 5, 180159. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.; Zhang, H.; Li, H.; Gao, Y.; Wang, Z.; Wang, H.; Li, L.; Zhang, C.; Chen, C.; et al. Synergistic Anticancer Effect of RNAi and Photothermal Therapy Mediated by Functionalized Single-Walled Carbon Nanotubes. Biomaterials 2013, 34, 262–274. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Martinelli, V.; Bosi, S.; Peña, B.; Baj, G.; Long, C.S.; Sbaizero, O.; Giacca, M.; Prato, M.; Mestroni, L. 3D Carbon-Nanotube-Based Composites for Cardiac Tissue Engineering. ACS Appl. Bio Mater. 2018, 1, 1530–1537. [Google Scholar] [CrossRef]
- Mamidi, N.; Leija, H.M.; Diabb, J.M.; Lopez Romo, I.; Hernandez, D.; Castrejón, J.V.; Martinez Romero, O.; Barrera, E.V.; Elias Zúñiga, A. Cytotoxicity Evaluation of Unfunctionalized Multiwall Carbon Nanotubes-Ultrahigh Molecular Weight Polyethylene Nanocomposites. J. Biomed. Mater. Res. A 2017, 105, 3042–3049. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Vashist, A.; Sagar, V.; Ghosal, A.; Gupta, Y.K.; Ahmad, S.; Nair, M. Advances in Carbon Nanotubes-Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv. Healthc. Mater. 2018, 7, 1701213. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Cirillo, G.; Hampel, S.; Spizzirri, U.G.; Parisi, O.I.; Picci, N.; Iemma, F. Carbon Nanotubes Hybrid Hydrogels in Drug Delivery: A Perspective Review. BioMed Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Park, S.; Won, K.; Kim, H.J.; Lee, S.H. Bacterial Cellulose-Carbon Nanotube Composite as a Biocompatible Electrode for the Direct Electron Transfer of Glucose Oxidase: Biocompatible Electrode for Direct Electron Transfer of Glucose Oxidase. J. Chem. Technol. Biotechnol. 2013, 88, 1067–1070. [Google Scholar] [CrossRef]
- Comba, F.N.; Romero, M.R.; Garay, F.S.; Baruzzi, A.M. Mucin and Carbon Nanotube-Based Biosensor for Detection of Glucose in Human Plasma. Anal. Biochem. 2018, 550, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Fatoni, A.; Numnuam, A.; Kanatharana, P.; Limbut, W.; Thammakhet, C.; Thavarungkul, P. A Highly Stable Oxygen-Independent Glucose Biosensor Based on a Chitosan-Albumin Cryogel Incorporated with Carbon Nanotubes and Ferrocene. Sens. Actuators B Chem. 2013, 185, 725–734. [Google Scholar] [CrossRef]
- Barone, P.W.; Yoon, H.; Ortiz-García, R.; Zhang, J.; Ahn, J.-H.; Kim, J.-H.; Strano, M.S. Modulation of Single-Walled Carbon Nanotube Photoluminescence by Hydrogel Swelling. ACS Nano 2009, 3, 3869–3877. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, T.H.; Lima, M.D.; Baughman, R.H.; Kim, S.J. Biothermal Sensing of a Torsional Artificial Muscle. Nanoscale 2016, 8, 3248–3253. [Google Scholar] [CrossRef]
- Lee, J.; Ko, S.; Kwon, C.H.; Lima, M.D.; Baughman, R.H.; Kim, S.J. Carbon Nanotube Yarn-Based Glucose Sensing Artificial Muscle. Small 2016, 12, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Kokabi, M.; Mousavi, S.M. Conductive Bacterial Cellulose/Multiwall Carbon Nanotubes Nanocomposite Aerogel as a Potentially Flexible Lightweight Strain Sensor. Carbohydr. Polym. 2018, 201, 228–235. [Google Scholar] [CrossRef]
- Andrews, R.; Weisenberger, M.C. Carbon Nanotube Polymer Composites. Curr. Opin. Solid State Mater. Sci. 2004, 8, 31–37. [Google Scholar] [CrossRef]
- Yildirim, E.D.; Yin, X.; Nair, K.; Sun, W. Fabrication, Characterization and Biocompatibility of Single-Walled Carbon Nanotube-Reinforced Alginate Composite Scaffolds Manufactured Using Freeform Fabrication Technique. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87B, 406–414. [Google Scholar] [CrossRef]
- Shin, S.R.; Bae, H.; Cha, J.M.; Mun, J.Y.; Chen, Y.-C.; Tekin, H.; Shin, H.; Farshchi, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon Nanotube Reinforced Hybrid Microgels as Scaffold Materials for Cell Encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef]
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Ostrovidov, S.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Bae, H.; et al. Hybrid Hydrogels Containing Vertically Aligned Carbon Nanotubes with Anisotropic Electrical Conductivity for Muscle Myofiber Fabrication. Sci. Rep. 2014, 4, 4271. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Yamada, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Hybrid Hydrogel-Aligned Carbon Nanotube Scaffolds to Enhance Cardiac Differentiation of Embryoid Bodies. Acta Biomater. 2016, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhao, H.; Huang, C.; Du, Y. Mechanically and Electrically Enhanced CNT–Collagen Hydrogels As Potential Scaffolds for Engineered Cardiac Constructs. ACS Biomater. Sci. Eng. 2017, 3, 3017–3021. [Google Scholar] [CrossRef]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible Carbon Nanotube–Chitosan Scaffold Matching the Electrical Conductivity of the Heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.G.; Im, O.; Li, J.; Keidar, M. Biomimetic Three-Dimensional Nanocrystalline Hydroxyapatite and Magnetically Synthesized Single-Walled Carbon Nanotube Chitosan Nanocomposite for Bone Regeneration. Int. J. Nanomed. 2012, 7, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Cancian, G.; Tozzi, G.; Hussain, A.A.B.; De Mori, A.; Roldo, M. Carbon Nanotubes Play an Important Role in the Spatial Arrangement of Calcium Deposits in Hydrogels for Bone Regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 126. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, J.C.; Miller, A.L.; Waletzki, B.E.; Lu, L. Electrically Conductive Nanocomposite Hydrogels Embedded with Functionalized Carbon Nanotubes for Spinal Cord Injury. New J. Chem. 2018, 42, 17671–17681. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Wheeler, S.D.; Duke, K.G.; Marsden, M.; Pritzker, M.; Chen, P. Peptide and Peptide-Carbon Nanotube Hydrogels as Scaffolds for Tissue & 3D Tumor Engineering. Acta Biomater. 2018, 69, 107–119. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Zhao, Y.; Wang, G.; Wei, Q. The Effect of Carbon Nanotubes Added into Bullfrog Collagen Hydrogel on Gentamicin Sulphate Release: In Vitro. J. Inorg. Organomet. Polym. Mater. 2011, 21, 890–892. [Google Scholar] [CrossRef]
- Cirillo, G.; Vittorio, O.; Hampel, S.; Iemma, F.; Parchi, P.; Cecchini, M.; Puoci, F.; Picci, N. Quercetin Nanocomposite as Novel Anticancer Therapeutic: Improved Efficiency and Reduced Toxicity. Eur. J. Pharm. Sci. 2013, 49, 359–365. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Hampel, S.; Cirillo, G.; Nicoletta, F.P.; Hassan, A.; Vittorio, O.; Picci, N.; Iemma, F. Spherical Gelatin/CNTs Hybrid Microgels as Electro-Responsive Drug Delivery Systems. Int. J. Pharm. 2013, 448, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Picci, N.; Nicoletta, F.P.; Iemma, F.; Hampel, S. On Demand Delivery of Ionic Drugs from Electro-Responsive CNT Hybrid Films. RSC Adv. 2015, 5, 44902–44911. [Google Scholar] [CrossRef]
- Servant, A.; Bussy, C.; Al-Jamal, K.; Kostarelos, K. Design, Engineering and Structural Integrity of Electro-Responsive Carbon Nanotube- Based Hydrogels for Pulsatile Drug Release. J. Mater. Chem. B 2013, 1, 4593. [Google Scholar] [CrossRef]
- Servant, A.; Methven, L.; Williams, R.P.; Kostarelos, K. Electroresponsive Polymer-Carbon Nanotube Hydrogel Hybrids for Pulsatile Drug Delivery In Vivo. Adv. Healthc. Mater. 2013, 2, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhuang, Q.; Peng, D.; Dong, Q.; Tan, L.; Jiao, F.; Liu, L. Sustained Release of Naproxen in a New Kind Delivery System of Carbon Nanotubes Hydrogel. Iran. J. Pharm. Res. 2013, 12, 581–586. [Google Scholar]
- Ye, Y.; Mao, Y.; Wang, H.; Ren, Z. Hybrid Structure of PH-Responsive Hydrogel and Carbon Nanotube Array with Superwettability. J. Mater. Chem. 2012, 22, 2449–2455. [Google Scholar] [CrossRef]
- Wei, C.; Dong, X.; Zhang, Y.; Liang, J.; Yang, A.; Zhu, D.; Liu, T.; Kong, D.; Lv, F. Simultaneous Fluorescence Imaging Monitoring of the Programmed Release of Dual Drugs from a Hydrogel-Carbon Nanotube Delivery System. Sens. Actuators B Chem. 2018, 273, 264–275. [Google Scholar] [CrossRef]
- Hindumathi, R.; Jagannatham, M.; Haridoss, P.; Sharma, C.P. Novel Nano-Cocoon like Structures of Polyethylene Glycol–Multiwalled Carbon Nanotubes for Biomedical Applications. Nano-Struct. Nano-Objects 2018, 13, 30–35. [Google Scholar] [CrossRef]
- Kuche, K.; Maheshwari, R.; Tambe, V.; Mak, K.-K.; Jogi, H.; Raval, N.; Pichika, M.R.; Kumar Tekade, R. Carbon Nanotubes (CNTs) Based Advanced Dermal Therapeutics: Current Trends and Future Potential. Nanoscale 2018, 10, 8911–8937. [Google Scholar] [CrossRef]
- Im, J.S.; Bai, B.C.; Lee, Y.-S. The Effect of Carbon Nanotubes on Drug Delivery in an Electro-Sensitive Transdermal Drug Delivery System. Biomaterials 2010, 31, 1414–1419. [Google Scholar] [CrossRef]
- Bhunia, T.; Giri, A.; Nasim, T.; Chattopadhyay, D.; Bandyopadhyay, A. A Transdermal Diltiazem Hydrochloride Delivery Device Using Multi-Walled Carbon Nanotube/Poly(Vinyl Alcohol) Composites. Carbon 2013, 52, 305–315. [Google Scholar] [CrossRef]
- Giri, A.; Bhunia, T.; Mishra, S.R.; Goswami, L.; Panda, A.B.; Bandyopadhyay, A. A Transdermal Device from 2-Hydroxyethyl Methacrylate Grafted Carboxymethyl Guar Gum–Multi-Walled Carbon Nanotube Composites. RSC Adv. 2014, 4, 13546. [Google Scholar] [CrossRef]
- Guillet, J.-F.; Flahaut, E.; Golzio, M. A Hydrogel/Carbon-Nanotube Needle-Free Device for Electrostimulated Skin Drug Delivery. ChemPhysChem 2017, 18, 2715–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, F.; Billups, E. Water-Soluble Single-Wall Carbon Nanotubes as a Platform Technology for Bomedical Applications. U.S. Patent 2007/0110658 A1, 17 May 2007. [Google Scholar]
- Harmon, J.P.; Clayton, L.M. Polymer/Carbon Nanotube Composites, Methods of Use and Methods of Synthesis Thereof. U.S. Patent 7,754,055 B2, 13 July 2010. [Google Scholar]
- Sheng, X.H.; Coates, D.; Dong, L.; Xia, F.G.; Chung, G.Q. Methods to Improve the Electrical Conductivity for Moulded Plastic Parts. U.S. Patent 2014/0001415 A1, 2 January 2014. [Google Scholar]
- Wallace, G.G.; Chen, J.; Minett, A.I.; Clark, G.M. Nanostructured Composites. U.S. Patent 2010/0068461 A1, 18 March 2010. [Google Scholar]
- Argenta, L.; Levi, N.H.; Morykwas, M.; Walles, E.; McGee, M.; Wagner, W.D.; Graham, E. Methods and Compositions for Inhibiting Fibrosis, Scarring and/or Fbrotc Contractures. U.S. Patent 2015/0367031 A1, 24 December 2015. [Google Scholar]
- Ko, F.K.; Sukigara, S.; Gandhi, M.; Ayutsede, J. Electrospun Carbon Nanotube Reinforced Silk Fibers. U.S. Patent 2007/0082197 A1, 12 April 2007. [Google Scholar]
- Harmon, J.P.; Muisener, P.A.O.; Clayton, L.M.; D’Angelo, J. Carbon Nanotubeapolymer Composites Resistant to Ionzng Radation. U.S. Patent 8,815,144 B2, 26 October 2014. [Google Scholar]
- Li, G.; Liao, J.M.; Hu, G.Q.; Ma, N.Z.; Wu, P.J. Study of Carbon Nanotube Modified Biosensor for Monitoring Total Cholesterol in Blood. Biosens. Bioelectron. 2005, 20, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, E.; Evariste, L.; Gauthier, L.; Larue, C.; Line, C.; Meunier, É.; Mouchet, F. Toxicité des nanotubes de carbone envers l’homme et l’environnement. Tech. L’ingénieur 2018, 22, NM8155 v1. [Google Scholar]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A Concise Review of Carbon Nanotube’s Toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-F.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.-S. Interfacing Carbon Nanotubes with Living Mammalian Cells and Cytotoxicity Issues. Chem. Res. Toxicol. 2010, 23, 1131–1147. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Kostarelos, K. Pharmacology of Carbon Nanotubes: Toxicokinetics, Excretion and Tissue Accumulation. Adv. Drug Deliv. Rev. 2013, 65, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Georgin, D.; Czarny, B.; Botquin, M.; Mayne-L’Hermite, M.; Pinault, M.; Bouchet-Fabre, B.; Carriere, M.; Poncy, J.-L.; Chau, Q.; Maximilien, R.; et al. Preparation of 14C-Labeled Multiwalled Carbon Nanotubes for Biodistribution Investigations. J. Am. Chem. Soc. 2009, 131, 14658–14659. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Fabbro, C.; Venturelli, E.; Ménard-Moyon, C.; Chaloin, O.; Da Ros, T.; Methven, L.; Nunes, A.; Sosabowski, J.K.; Mather, S.J.; et al. The Relationship between the Diameter of Chemically-Functionalized Multi-Walled Carbon Nanotubes and Their Organ Biodistribution Profiles in Vivo. Biomaterials 2014, 35, 9517–9528. [Google Scholar] [CrossRef]
- Czarny, B.; Georgin, D.; Berthon, F.; Plastow, G.; Pinault, M.; Patriarche, G.; Thuleau, A.; L’Hermite, M.M.; Taran, F.; Dive, V. Carbon Nanotube Translocation to Distant Organs after Pulmonary Exposure: Insights from in Situ 14C-Radiolabeling and Tissue Radioimaging. ACS Nano 2014, 8, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms and Remedies of Toxicity: Miniperspective. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef] [PubMed]
- Meunier, É.; Coste, A.; Olagnier, D.; Auhtier, H.; Lefèvre, L.; Dardenne, C.; Bernad, J.; Béraud, M.; Flahaut, E.; Pipy, B. Double-Walled Carbon Nanotubes Trigger IL-1β Release in Human Monocytes through Nlrp3 Inflammasome Activation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, D.; Follot, S.; Gentilhomme, E.; Flahaut, E.; Arnaud, R.; Dabouis, V.; Castellarin, C.; Debouzy, J.C. Carbon Nanotubes Induce Inflammation but Decrease the Production of Reactive Oxygen Species in Lung. Toxicology 2010, 272, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K. The Long and Short of Carbon Nanotube Toxicity. Nat. Biotechnol. 2008, 26, 774–776. [Google Scholar] [CrossRef]
- Pumera, M. Nanotoxicology: The Molecular Science Point of View. Chem. Asian J. 2011, 6, 340–348. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron Metabolism and Toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.; Yanamala, N.V.K.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic Investigations of Horseradish Peroxidase-Catalyzed Degradation of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef]
- Mottier, A.; Mouchet, F.; Laplanche, C.; Cadarsi, S.; Lagier, L.; Arnault, J.-C.; Girard, H.; Leon, V.; Vazquez, E.; Sarrieu, C.; et al. Surface Area of Carbon Nanoparticles: A Dose Metric for a More Realistic Ecotoxicological Assessment. Nano Lett. 2016, 16, 3514–3518. [Google Scholar] [CrossRef]
- Bellingeri, R.; Alustiza, F.; Picco, N.; Acevedo, D.; Molina, M.A.; Rivero, R.; Grosso, C.; Motta, C.; Barbero, C.; Vivas, A. In Vitro Toxicity Evaluation of Hydrogel-Carbon Nanotubes Composites on Intestinal Cells. J. Appl. Polym. Sci. 2015, 132, 41370. [Google Scholar] [CrossRef]






| Bio-Sourced Polymers | Synthetic Monomers |
|---|---|
| Agarose | Acrylamide (AM) |
| Alginate | Acrylic acid (AA) |
| Chitosan | Ethylene glycol (EG) |
| Collagen | Hydroxyethyl methacrylate (HEMA) |
| Dextran | Lactic acid (LA) |
| Fibrin | Methyl methacrylate (MMA) |
| Hyaluronic acid | Vinyl alcohol (VA) |
| Pectin | - |
| CNT | CNT-Based Hydrogels | ||
|---|---|---|---|
| Biosensors | [15,16,17,18,19,20,21,22,23] | Diagnostic | [72,73,74,75,76,77,78] |
| Imaging | [24,25,26,27,28,29,30,31,32,33,34] | ||
| Cells guidance and tissue scaffolds | [35,36,37,38,39,40,41] | Cells guidance and tissue scaffolds | [79,80,81,82,83,84,85,86,87,88,89] |
| Drugs and gene delivery | [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56] | Drugs delivery | [71,90,91,92,93,94,95,96,97,98,99] |
| Anticancer therapies | [44,57,58,59,60,61] | Skin delivery | [100,101,102,103,104] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. https://doi.org/10.3390/ma12040624
Simon J, Flahaut E, Golzio M. Overview of Carbon Nanotubes for Biomedical Applications. Materials. 2019; 12(4):624. https://doi.org/10.3390/ma12040624
Chicago/Turabian StyleSimon, Juliette, Emmanuel Flahaut, and Muriel Golzio. 2019. "Overview of Carbon Nanotubes for Biomedical Applications" Materials 12, no. 4: 624. https://doi.org/10.3390/ma12040624
APA StyleSimon, J., Flahaut, E., & Golzio, M. (2019). Overview of Carbon Nanotubes for Biomedical Applications. Materials, 12(4), 624. https://doi.org/10.3390/ma12040624