Effects of Alkali Metal (Li, Na, and K) Incorporation in NH2–MIL125(Ti) on the Performance of CO2 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of NH2–MIL125(Ti)
2.3. Synthesis of xM@NH2–MIL125(Ti)
2.4. Sample Characterization
3. Results
3.1. FT-IR Analysis
3.2. X-ray Diffraction Analysis
3.3. X-ray Photoelectron Spectrometer
3.4. Thermal Stability
3.5. Scanning Electron Microscopy
3.6. Alkali Metal Content
3.7. N2 Asorption Isotherms
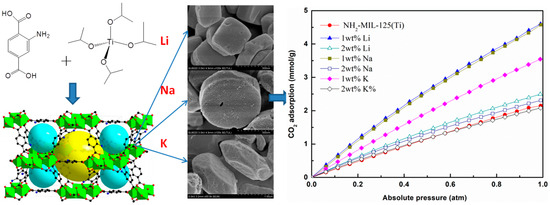
3.8. CO2 Adsorption Isotherms Measured
3.9. CO2 Adsorption Regenerability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandez-Martinez, M.; Sardans, J.; Chevallier, F.; Ciais, P.; Obersteiner, M.; Vicca, S.; Canadell, J.G.; Bastos, A.; Friedlingstein, P.; Sitch, S.; et al. Global trends in carbon sinks and their relationships with CO2 and temperature. Nat. Clim. Chang. 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.A.S.; Bezerra, D.P.; Vilarrasa-Garcia, E.; Bastos-Neto, M.; Azevedo, D.C.S. Adsorption equilibria of CO2 and CH4 in cation-exchanged zeolites 13X. Adsorption 2016, 22, 71–80. [Google Scholar] [CrossRef]
- Sanchez-Zambrano, K.S.; Lima Duarte, L.; Maia, D.A.S.; Vilarrasa-Garcia, E.; Bastos-Neto, M.; Rodriguez-Castellon, E.; de Azevedo, D.C.S. CO2 Capture with Mesoporous Silicas Modified with Amines by Double Functionalization: Assessment of Adsorption/Desorption Cycles. Materials 2018, 11, 887. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.Q.; Wu, Z.J.; Luo, X.Y.; Zhuang, L.Z.; Ou, H.Z.; Chen, S.X. Synthesis of nitrogen-rich hollow microspheres for CO2 adsorption. J. Mater. Sci. 2019, 54, 3805–3816. [Google Scholar] [CrossRef]
- Jin, C.N.; Zhang, S.N.; Zhang, Z.J.; Chen, Y. Mimic Carbonic Anhydrase Using Metal-Organic Frameworks for CO2 Capture and Conversion. Inorg. Chem. 2018, 57, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, Y.X.; Song, F.J.; Zhong, Q. Alkali metal cation doping of metal-organic framework for enhancing carbon dioxide adsorption capacity. J. Energy Chem. 2014, 23, 468–474. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Z.W.; Du, X.Y.; Zhang, S.Q.; Chen, Z.B. Lanthanum doping of metal-organic frameworks-5 and its effect on thermal stability and CO2 adsorption property. Mater. Express 2018, 8, 381–387. [Google Scholar] [CrossRef]
- Wen, H.M.; Li, L.B.; Lin, R.B.; Li, B.; Hu, B.; Zhou, W.; Hu, J.; Chen, B. Fine-tuning of nano-traps in a stable metal-organic framework for highly efficient removal of propyne from propylene. J. Mater. Chem. A 2018, 6, 6931–6937. [Google Scholar] [CrossRef]
- Rada, Z.H.; Abid, H.R.; Shang, J.; He, Y.D.; Webley, P.; Liu, S.M.; Sun, H.; Wang, S. Effects of amino functionality on uptake of CO2, CH4 and selectivity of CO2/CH4 on titanium based MOFs. Fuel 2015, 160, 318–327. [Google Scholar] [CrossRef]
- Vaidhyanathan, R.; Iremonger, S.S.; Shimizu, G.K.H.; Boyd, P.G.; Alavi, S.; Woo, T.K. Direct Observation and Quantification of CO2 Binding within an Amine-Functionalized Nanoporous Solid. Science 2010, 330, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, H.M.; Song, F.J.; Huang, T.; Ji, J.Y.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials 2018, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.H.; Cao, D.P.; Wang, W.C. High Uptakes of Methane in Li-Doped 3D Covalent Organic Frameworks. Langmuir 2010, 26, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.P.; Lan, J.H.; Wang, W.C.; Smit, B. Lithium-Doped 3D Covalent Organic Frameworks: High-Capacity Hydrogen Storage Materials. Angew. Chem. Int. Ed. 2009, 48, 4730–4733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, W.; Liu, D.H.; Zhong, C.L. A computational study of the effect of doping metals on CO2/CH4 separation in metal-organic frameworks. Microporous Mesoporous Mater. 2011, 143, 66–72. [Google Scholar] [CrossRef]
- Babarao, R.; Jiang, J.W. Molecular screening of metal-organic frameworks for CO2 storage. Langmuir 2008, 24, 6270–6278. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.H.; Hu, Z.; Cao, D.P.; Yang, W.T.; Lu, J.M.; Han, B.Y.; Wang, W. Metal-Organic Frameworks with Incorporated Carbon Nanotubes: Improving Carbon Dioxide and Methane Storage Capacities by Lithium Doping. Angew. Chem. Int. Edit. 2011, 50, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.Q.; Jiang, H.L.; Sun, L.B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Huh, S. Pore engineering of metal-organic frameworks: Introduction of chemically accessible Lewis basic sites inside MOF channels. CrystEngComm 2016, 18, 3524–3550. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, R.J.; Ge, L.; Hou, L.; Bernhardt, P.; Rufford, T.E.; Wang, S.; Rudolph, V.; Wang, Y.-Y.; Zhu, Z. Synthesis and characterization of three amino-functionalized metal-organic frameworks based on the 2-aminoterephthalic ligand. Dalton Trans. 2015, 44, 8190–8197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Chen, Z.J.; Yang, X.Q.; Li, M.Y.; Chen, C.; Zhang, N. The fixation of carbon dioxide with epoxides catalyzed by cation-exchanged metal-organic framework. Microporous Mesoporous Mater. 2018, 258, 55–61. [Google Scholar] [CrossRef]
- Kim, S.N.; Kim, J.; Kim, H.Y.; Cho, H.Y.; Ahn, W.S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Sohail, M.; Yun, Y.N.; Lee, E.; Kim, S.K.; Cho, K.; Kim, J.N.; Kim, T.W.; Moon, J.H.; Kim, H. Synthesis of Highly Crystalline NH2-MIL-125 (Ti) with S-Shaped Water Isotherms for Adsorption Heat Transformation. Cryst Growth Des. 2017, 17, 1208–1213. [Google Scholar] [CrossRef]
- Nasalevich, M.A.; Becker, R.; Ramos-Fernandez, E.V.; Castellanos, S.; Veber, S.L.; Fedin, M.V.; Kapteijn, F.; Reek, J.N.H.; van der Vlugt, J.I.; Gascon, J. Co@NH2-MIL-125(Ti): Cobaloxime-derived metal-organic framework-based composite for light-driven H-2 production. Energy Environ. Sci. 2015, 8, 364–375. [Google Scholar] [CrossRef]
- Martis, M.; Meicheng, W.; Mori, K.; Yamashit, H. Fabrication of metal nanoparticles in metal organic framework NH2-MIL-125 by UV photo-assisted methods for optimized catalytic properties. Catal. Today 2014, 235, 98–102. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Li, K.Y.; Zuo, Y.; Zhang, A.F.; Song, C.S.; Zhang, G.L.; Guo, X.W. Solvothermal synthesis of NH2-MIL-125(Ti) from circular plate to octahedron. Crystengcomm 2014, 16, 9645–9650. [Google Scholar] [CrossRef]
- Chaemchuem, S.; Kui, Z.; Verpoort, F. Control of interpenetration via in situ lithium incorporation in MOFs and their gas adsorption properties and selectivity. CrystEngComm 2016, 18, 7614–7619. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.Z.; Wu, Y.; Zeng, G.M.; Chen, X.H.; Leng, L.J.; Wu, Z.B.; Jiang, L.B.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef]
- Hu, S.; Liu, M.; Guo, X.W.; Kuang, Z.C.; Li, K.Y.; Song, C.S. Effect of titanium ester on synthesizing NH2-MIL-125(Ti): Morphology changes from circular plate to octahedron and rhombic dodecahedron. J. Solid State Chem. 2018, 262, 237–243. [Google Scholar] [CrossRef]
- Lu, J.T.; Jiao, C.L.; Majeed, Z.; Jiang, H.Q. Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption. Nanomaterials 2018, 8, 275. [Google Scholar] [CrossRef]
- Daliran, S.; Santiago-Portillo, A.; Navalon, S.; Oveisi, A.R.; Alvaro, M.; Ghorbani-Vaghei, R.; Azarifar, D.; Garcia, H. Cu(II)-Schiff base covalently anchored to MIL-125(Ti)-NH2 as heterogeneous catalyst for oxidation reactions. J. Colloid Interfaces Sci. 2018, 532, 700–710. [Google Scholar] [CrossRef]
- Anbia, M.; Hoseini, V. Enhancement of CO2 adsorption on nanoporous chromium terephthalate (MIL-101) by amine modification. J. Nat. Gas Chem. 2012, 21, 339–343. [Google Scholar] [CrossRef]
- Molavi, H.; Eskandari, A.; Shojaei, A.; Mousavi, S.A. Enhancing CO2/N2 adsorption selectivity via post-synthetic modification of NH2-UiO-66(Zr). Microporous Mesoporous Mater. 2018, 257, 193–201. [Google Scholar] [CrossRef]
- Hong, D.H.; Suh, M.P. Enhancing CO2 Separation Ability of a Metal-Organic Framework by Post-Synthetic Ligand Exchange with Flexible Aliphatic Carboxylates. Chem. Eur. J. 2014, 20, 426–434. [Google Scholar] [CrossRef]
- Yang, D.A.; Cho, H.Y.; Kim, J.; Yang, S.T.; Ahn, W.S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 2012, 5, 6465–6473. [Google Scholar] [CrossRef]











| Samples | SBET (m2 g−1) | CO2 Adsorption (mmol g−1) | Alkali Metal Introduced in the Synthesis (wt %) | Metal Analysis (ICP, wt %) | |
|---|---|---|---|---|---|
| Ti | Alkali Metal | ||||
| NH2–MIL125(Ti) | 1038 | 2.13 | 0 | 16.3 | 0 |
| 1Li@NH2–MIL125(Ti) | 1470 | 4.60 | 0.164 | 16.7 | 0.082 |
| 2Li@NH2–MIL125(Ti) | 1115 | 2.31 | 0.327 | 16.1 | 0.099 |
| 1Na@NH2–MIL125(Ti) | 1451 | 4.57 | 0.393 | 15.7 | 0.065 |
| 2Na@NH2–MIL125(Ti) | 985 | 2.50 | 0.787 | 16.9 | 0.118 |
| 1K@NH2–MIL125(Ti) | 1226 | 3.55 | 0.524 | 15.6 | 0.378 |
| 2K@NH2–MIL125(Ti) | 872 | 2.08 | 1.049 | 15.8 | 0.456 |
| MOFs | Condition | CO2 Adsorption (mmol g−1) | References |
|---|---|---|---|
| PEHA-MIL-101 | 298 K, 10 bar | 1.30 | [33] |
| MIL-101(Cr) | 298 K, 10 bar | 0.85 | [33] |
| NH2-UiO-66 | 298 K, 1 bar | 3.15 | [34] |
| UiO-66-AD6 | 298 K, 1 bar | 2.63 | [35] |
| Mg-MOF-74 | 298 K, 1 bar | 7.95 | [36] |
| MIL125(Ti) | 298 K, 1 bar | 3.00 | [11] |
| NH2-MIL125(Ti) | 298 K, 1 bar | 2.18 | [11] |
| NH2-MIL125(Ti) | 293 K, 1 bar | 2.13 | This work |
| 1Li@NH2-MIL125(Ti) | 293 K, 1 bar | 4.60 | This work |
| 1Na@NH2-MIL125(Ti) | 293 K, 1 bar | 4.57 | This work |
| 1K@NH2-MIL125(Ti) | 293 K, 1 bar | 3.55 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Xue, C.; Xia, H.; Qiu, S.; Sun, L.; Chen, H. Effects of Alkali Metal (Li, Na, and K) Incorporation in NH2–MIL125(Ti) on the Performance of CO2 Adsorption. Materials 2019, 12, 844. https://doi.org/10.3390/ma12060844
Song L, Xue C, Xia H, Qiu S, Sun L, Chen H. Effects of Alkali Metal (Li, Na, and K) Incorporation in NH2–MIL125(Ti) on the Performance of CO2 Adsorption. Materials. 2019; 12(6):844. https://doi.org/10.3390/ma12060844
Chicago/Turabian StyleSong, Lifang, Cheng Xue, Huiyun Xia, Shujun Qiu, Lixian Sun, and Huaxin Chen. 2019. "Effects of Alkali Metal (Li, Na, and K) Incorporation in NH2–MIL125(Ti) on the Performance of CO2 Adsorption" Materials 12, no. 6: 844. https://doi.org/10.3390/ma12060844
APA StyleSong, L., Xue, C., Xia, H., Qiu, S., Sun, L., & Chen, H. (2019). Effects of Alkali Metal (Li, Na, and K) Incorporation in NH2–MIL125(Ti) on the Performance of CO2 Adsorption. Materials, 12(6), 844. https://doi.org/10.3390/ma12060844