High-κ Dielectric on ReS2: In-Situ Thermal Versus Plasma-Enhanced Atomic Layer Deposition of Al2O3
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Surface Analysis of the Exfoliated ReS2 Surface
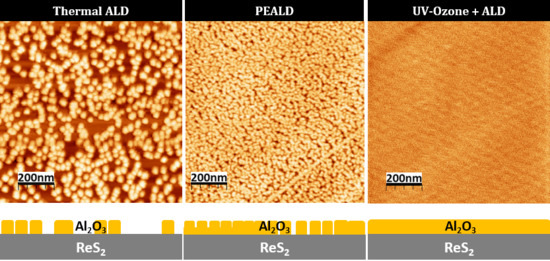
Thermal ALD of Al2O3 on ReS2
3.2. Plasma-Enhanced ALD of Al2O3 on ReS2
3.3. Al2O3 on A UV-Ozone Exposed ReS2 Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amani, M.; Chin, M.L.; Birdwell, A.G.; O’Regan, T.P.; Najmaei, S.; Liu, Z.; Ajayan, P.M.; Lou, J.; Dubey, M. Electrical performance of monolayer MoS2 field-effect transistors prepared by chemical vapor deposition. Appl. Phys. Lett. 2013, 102, 193107. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Zubair, A.; Sajjad, R.N.; Tavakkoli KG, A.; Chen, W.; Fang, S.; Ling, X.; Kong, J.; Dresselhaus, M.S.; Kaxiras, E. MoS2 field-effect transistor with sub-10 nm channel length. Nano Lett. 2016, 16, 7798–7806. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699. [Google Scholar] [CrossRef] [PubMed]
- Tongay, S.; Sahin, H.; Ko, C.; Luce, A.; Fan, W.; Liu, K.; Zhou, J.; Huang, Y.-S.; Ho, C.-H.; Yan, J. Monolayer behaviour in bulk ReS2 due to electronic and vibrational decoupling. Nat. Commun. 2014, 5, 3252. [Google Scholar] [CrossRef] [PubMed]
- Horzum, S.; Çakır, D.; Suh, J.; Tongay, S.; Huang, Y.-S.; Ho, C.-H.; Wu, J.; Sahin, H.; Peeters, F. Formation and stability of point defects in monolayer rhenium disulfide. Phys. Rev. B 2014, 89, 155433. [Google Scholar] [CrossRef]
- Yu, Z.G.; Cai, Y.; Zhang, Y.-W. Robust Direct Bandgap Characteristics of One-and Two-Dimensional ReS2. Sci. Rep. 2015, 5, 13783. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.-Q.; Zhang, S.-F.; Ji, W.-X.; Zhang, C.-W.; Li, P.; Wang, P.-J. Electric structure and optical properties of ReS2 nanomaterials. Superlattice Microst. 2018, 122, 262–267. [Google Scholar] [CrossRef]
- Echeverry, J.; Gerber, I. Theoretical investigations of the anisotropic optical properties of distorted 1T ReS2 and ReSe2 monolayers, bilayers, and in the bulk limit. Phys. Rev. B 2018, 97, 075123. [Google Scholar] [CrossRef]
- Gutiérrez-Lezama, I.; Reddy, B.A.; Ubrig, N.; Morpurgo, A.F. Electroluminescence from indirect band gap semiconductor ReS2. 2D Mater. 2016, 3, 045016. [Google Scholar] [CrossRef]
- Webb, J.L.; Hart, L.S.; Wolverson, D.; Chen, C.; Avila, J.; Asensio, M.C. Electronic band structure of ReS2 by high-resolution angle-resolved photoemission spectroscopy. Phys. Rev. B 2017, 96, 115205. [Google Scholar] [CrossRef]
- Gehlmann, M.; Aguilera, I.; Bihlmayer, G.; Nemsák, S.; Nagler, P.; Gospodaric, P.; Zamborlini, G.; Eschbach, M.; Feyer, V.; Kronast, F. Direct observation of the band gap transition in atomically thin ReS2. Nano Lett. 2017, 17, 5187–5192. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.M.; Wolverson, D.; Hart, L.S.; Mucha-Kruczynski, M. Electronic band structure of rhenium dichalcogenides. J. Electron. Mater. 2018, 47, 1–7. [Google Scholar] [CrossRef]
- Hart, L.S.; Webb, J.L.; Dale, S.; Bending, S.J.; Mucha-Kruczynski, M.; Wolverson, D.; Chen, C.; Avila, J.; Asensio, M.C. Electronic bandstructure and van der Waals coupling of ReSe2 revealed by high-resolution angle-resolved photoemission spectroscopy. Sci. Rep. 2017, 7, 5145. [Google Scholar] [CrossRef] [PubMed]
- Corbet, C.M.; McClellan, C.; Rai, A.; Sonde, S.S.; Tutuc, E.; Banerjee, S.K. Field effect transistors with current saturation and voltage gain in ultrathin ReS2. ACS Nano 2014, 9, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Jin, Y.; Yuan, X.; Wang, W.; Zhang, C.; Tang, L.; Liu, S.; Zhou, P.; Hu, W.; Xiu, F. ReS2-Based Field-Effect Transistors and Photodetectors. Adv. Funct. Mater. 2015, 25, 4076–4082. [Google Scholar] [CrossRef]
- He, X.; Liu, F.; Hu, P.; Fu, W.; Wang, X.; Zeng, Q.; Zhao, W.; Liu, Z. Chemical Vapor Deposition of High-Quality and Atomically Layered ReS2. Small 2015, 11, 5423–5429. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Oh, A.; Kang, D.H.; Oh, S.; Jang, S.K.; Jeon, J.; Jeon, M.H.; Kim, M.; Choi, C.; Lee, J. High-Performance 2D Rhenium Disulfide (ReS2) Transistors and Photodetectors by Oxygen Plasma Treatment. Adv. Mater. 2016, 28, 6985–6992. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Fu, Y.; Wang, Y.; Feng, Y.; Liu, H.; Wan, X.; Zhou, W.; Wang, B.; Shao, L.; Ho, C.-H. Integrated digital inverters based on two-dimensional anisotropic ReS2 field-effect transistors. Nat. Commun. 2015, 6, 6991. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Komsa, H.-P.; Yeh, C.-H.; Bjorkman, T.; Liang, Z.-Y.; Ho, C.-H.; Huang, Y.-S.; Chiu, P.-W.; Krasheninnikov, A.V.; Suenaga, K. Single-layer ReS2: Two-dimensional semiconductor with tunable in-plane anisotropy. ACS Nano 2015, 9, 11249–11257. [Google Scholar] [CrossRef]
- Liao, W.; Wei, W.; Tong, Y.; Chim, W.K.; Zhu, C. Low-Frequency Noise in Layered ReS2 Field Effect Transistors on HfO2 and Its Application for pH Sensing. ACS Appl. Mater. Interfaces 2018, 10, 7248–7255. [Google Scholar] [CrossRef]
- Park, J.Y.; Joe, H.-E.; Yoon, H.S.; Yoo, S.; Kim, T.; Kang, K.; Min, B.-K.; Jun, S.C. Contact effect of ReS2/metal interface. ACS Appl. Mater. Interfaces 2017, 9, 26325–26332. [Google Scholar] [CrossRef] [PubMed]
- Addou, R.; Wallace, R.M. Using Photoelectron Spectroscopy in the Integration of 2D Materials for Advanced Devices. J. Electron. Spectrosc. Relat. Phenom. 2019, 231, 94–103. [Google Scholar] [CrossRef]
- Jena, D.; Konar, A. Enhancement of carrier mobility in semiconductor nanostructures by dielectric engineering. Phys. Rev. Lett. 2007, 98, 136805. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.; Brennan, B.; Azcatl, A.; Lu, N.; Dong, H.; Buie, C.; Kim, J.; Hinkle, C.L.; Kim, M.J.; Wallace, R.M. HfO2 on MoS2 by atomic layer deposition: Adsorption mechanisms and thickness scalability. ACS Nano 2013, 7, 10354–10361. [Google Scholar] [CrossRef]
- Azcatl, A.; McDonnell, S.; KC, S.; Peng, X.; Dong, H.; Qin, X.; Addou, R.; Mordi, G.I.; Lu, N.; Kim, J. MoS2 functionalization for ultra-thin atomic layer deposited dielectrics. Appl. Phys. Lett. 2014, 104, 111601. [Google Scholar] [CrossRef]
- Azcatl, A.; Wang, Q.; Kim, M.J.; Wallace, R.M. Al2O3 on WSe2 by ozone based atomic layer deposition: Nucleation and interface study. APL Mater. 2017, 5, 086108. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, X.; Lucero, A.T.; Azcatl, A.; Huang, J.; Wallace, R.M.; Cho, K.; Kim, J. Atomic layer deposition of a high-k dielectric on MoS2 using trimethylaluminum and ozone. ACS Appl. Mater. Interfaces 2014, 6, 11834–11838. [Google Scholar] [CrossRef]
- Zhu, H.; McDonnell, S.; Qin, X.; Azcatl, A.; Cheng, L.; Addou, R.; Kim, J.; Ye, P.D.; Wallace, R.M. Al2O3 on black phosphorus by atomic layer deposition: An in-situ interface study. ACS Appl. Mater. Interfaces 2015, 7, 13038–13043. [Google Scholar] [CrossRef]
- Dahal, A.; Addou, R.; Azcatl, A.; Coy-Diaz, H.; Lu, N.; Peng, X.; de Dios, F.; Kim, J.; Kim, M.J.; Wallace, R.M. Seeding Atomic Layer Deposition of Alumina on Graphene with Yttria. ACS Appl. Mater. Interfaces 2015, 7, 2082–2087. [Google Scholar] [CrossRef]
- Addou, R.; Dahal, A.; Batzill, M. Growth of a two-dimensional dielectric monolayer on quasi-freestanding graphene. Nat. Nanotechnol. 2013, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.M. In-Situ Studies on 2D Materials. ECS Trans. 2014, 64, 109–116. [Google Scholar] [CrossRef]
- Herrera-Gomez, A.; Hegedus, A.; Meissner, P. Chemical depth profile of ultrathin nitrided SiO2 films. Appl. Phys. Lett. 2002, 81, 1014–1016. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gomez-Rodriguez, J.; Colchero, J.; Gómez-Herrero, J.; Baro, A. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Wang, J.; Shi, C.; Huang, J.; Zhang, H.; Liu, Q.; Liu, Q.; Zeng, H. Probing surface interactions of electrochemically active galena mineral surface using atomic force microscopy. J. Phys. Chem. C 2016, 120, 22433–22442. [Google Scholar] [CrossRef]
- Cui, F.; Wang, C.; Li, X.; Wang, G.; Liu, K.; Yang, Z.; Feng, Q.; Liang, X.; Zhang, Z.; Liu, S. Tellurium-Assisted Epitaxial Growth of Large-Area, Highly Crystalline ReS2 Atomic Layers on Mica Substrate. Adv. Mater. 2016, 28, 5019–5024. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sofer, Z.; Luxa, J.; Sedmidubský, D.; Ambrosi, A.; Pumera, M. Layered rhenium sulfide on free-standing three-dimensional electrodes is highly catalytic for the hydrogen evolution reaction: Experimental and theoretical study. Electrochem. Commun. 2016, 63, 39–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, S.; Mendes, R.G.; Sun, Z.; Chen, Y.; Kong, X.; Xue, Y.; Rümmeli, M.H.; Wu, X.; Chen, S. Extremely Weak van der Waals Coupling in Vertical ReS2 Nanowalls for High-Current-Density Lithium-Ion Batteries. Adv. Mater. 2016, 28, 2616–2623. [Google Scholar] [CrossRef]
- Fujita, T.; Ito, Y.; Tan, Y.; Yamaguchi, H.; Hojo, D.; Hirata, A.; Voiry, D.; Chhowalla, M.; Chen, M. Chemically exfoliated ReS2 nanosheets. Nanoscale 2014, 6, 12458–12462. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, B.; Voiry, D.; Jindal, A.; Chalke, B.A.; Bapat, R.; Thamizhavel, A.; Chhowalla, M.; Deshmukh, M.; Bhattacharya, A. Synthesis and characterization of ReS2 and ReSe2 layered chalcogenide single crystals. Chem. Mater. 2016, 28, 3352–3359. [Google Scholar] [CrossRef]
- Hafeez, M.; Gan, L.; Li, H.; Ma, Y.; Zhai, T. Large-Area Bilayer ReS2 Film/Multilayer ReS2 Flakes Synthesized by Chemical Vapor Deposition for High Performance Photodetectors. Adv. Funct. Mater. 2016, 26, 4551–4560. [Google Scholar] [CrossRef]
- Wei, J.; Bai, D.; Yang, L. Polymer photovoltaic cells with rhenium oxide as anode interlayer. PLoS ONE 2015, 10, e0133725. [Google Scholar] [CrossRef] [PubMed]
- Addou, R.; McDonnell, S.; Barrera, D.; Guo, Z.; Azcatl, A.; Wang, J.; Zhu, H.; Hinkle, C.L.; Quevedo-Lopez, M.; Alshareef, H.N. Impurities and electronic property variations of natural MoS2 crystal surfaces. ACS Nano 2015, 9, 9124–9133. [Google Scholar] [CrossRef]
- Keyshar, K.; Gong, Y.; Ye, G.; Brunetto, G.; Zhou, W.; Cole, D.P.; Hackenberg, K.; He, Y.; Machado, L.; Kabbani, M. Chemical vapor deposition of monolayer rhenium disulfide (ReS2). Adv. Mater. 2015, 27, 4640–4648. [Google Scholar] [CrossRef]
- Rahman, M.; Davey, K.; Qiao, S.Z. Advent of 2D rhenium disulfide (ReS2): Fundamentals to applications. Adv. Funct. Mater. 2017, 27, 1606129. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, W.; Wang, Y.; Zhou, J.; Liu, E.; Fu, Y.; Ni, Z.; Wu, X.; Yuan, H.; Miao, F. Raman vibrational spectra of bulk to monolayer ReS2 with lower symmetry. Phys. Rev. B 2015, 92, 054110. [Google Scholar] [CrossRef]
- Miao, P.; Qin, J.K.; Shen, Y.; Su, H.; Dai, J.; Song, B.; Du, Y.; Sun, M.; Zhang, W.; Wang, H.L. Unraveling the Raman Enhancement Mechanism on 1T′-Phase ReS2 Nanosheets. Small 2018, 14, 1704079. [Google Scholar] [CrossRef]
- Lee, B.C.; Na, J.; Choi, J.H.; Ji, H.; Kim, G.T.; Joo, M.K. Probing Distinctive Electron Conduction in Multilayer Rhenium Disulfide. Adv. Mater. 2018, 31, 1805860. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shinokita, K.; Lim, H.E.; Mohamed, N.B.; Miyauchi, Y.; Cuong, N.T.; Okada, S.; Matsuda, K. Direct and Indirect Exciton Dynamics in Few-Layered ReS2 Revealed by Photoluminescence and Pump-Probe Spectroscopy. Adv. Funct. Mater. 2018, 29, 1806169. [Google Scholar] [CrossRef]
- Pirkle, A.; McDonnell, S.; Lee, B.; Kim, J.; Colombo, L.; Wallace, R. The effect of graphite surface condition on the composition of Al2O3 by atomic layer deposition. Appl. Phys. Lett. 2010, 97, 082901. [Google Scholar] [CrossRef]
- Liu, D.; Hong, J.; Wang, X.; Li, X.; Feng, Q.; Tan, C.; Zhai, T.; Ding, F.; Peng, H.; Xu, H. Diverse Atomically Sharp Interfaces and Linear Dichroism of 1T’ ReS2-ReSe2 Lateral p-n Heterojunctions. Adv. Funct. Mater. 2018, 1804696. [Google Scholar] [CrossRef]
- Azcatl, A.; Qin, X.; Prakash, A.; Zhang, C.; Cheng, L.; Wang, Q.; Lu, N.; Kim, M.J.; Kim, J.; Cho, K. Covalent nitrogen doping and compressive strain in MoS2 by remote N2 plasma exposure. Nano Lett. 2016, 16, 5437–5443. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, S.; Kim, J.H.; Zhao, J.; Seok, J.; Keum, D.H.; Baik, J.; Choe, D.-H.; Chang, K.J.; Suenaga, K. Phase patterning for ohmic homojunction contact in MoTe2. Science 2015, 349, 625–628. [Google Scholar] [CrossRef]
- Khosravi, A.; Addou, R.; Smyth, C.M.; Yue, R.; Cormier, C.R.; Kim, J.; Hinkle, C.L.; Wallace, R.M. Covalent nitrogen doping in molecular beam epitaxy-grown and bulk WSe2. APL Mater. 2018, 6, 026603. [Google Scholar] [CrossRef]
- Price, K.M.; Schauble, K.E.; McGuire, F.A.; Farmer, D.B.; Franklin, A.D. Uniform Growth of Sub-5-Nanometer High-κ Dielectrics on MoS2 Using Plasma-Enhanced Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 23072–23080. [Google Scholar] [CrossRef]
- Hinkle, C.; Sonnet, A.; Vogel, E.; McDonnell, S.; Hughes, G.; Milojevic, M.; Lee, B.; Aguirre-Tostado, F.; Choi, K.; Kim, H. GaAs interfacial self-cleaning by atomic layer deposition. Appl. Phys. Lett. 2008, 92, 071901. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Khosravi, A.; Azcatl, A.; Bolshakov, P.; Mirabelli, G.; Caruso, E.; Hinkle, C.L.; Hurley, P.K.; Wallace, R.M.; Young, C.D. Evaluation of border traps and interface traps in HfO2/MoS2 gate stacks by capacitance-voltage analysis. 2D Mater. 2018, 5, 031002. [Google Scholar] [CrossRef]
- Yang, J.; Kim, S.; Choi, W.; Park, S.H.; Jung, Y.; Cho, M.-H.; Kim, H. Improved growth behavior of atomic-layer-deposited high-k dielectrics on multilayer MoS2 by oxygen plasma pretreatment. ACS Appl. Mater. Interfaces 2013, 5, 4739–4744. [Google Scholar] [CrossRef]
- Zou, X.; Wang, J.; Chiu, C.H.; Wu, Y.; Xiao, X.; Jiang, C.; Wu, W.W.; Mai, L.; Chen, T.; Li, J. Interface Engineering for High-Performance Top-Gated MoS2 Field-Effect Transistors. Adv. Mater. 2014, 26, 6255–6261. [Google Scholar] [CrossRef]
- McDonnell, S.J.; Wallace, R.M. UV-Ozone Functionalization of 2D Materials. JOM 2018, 1–14. [Google Scholar] [CrossRef]
- Wallace, R.M. In-Situ Studies of Interfacial Bonding of High-κ Dielectrics for CMOS Beyond 22 nm. Electrochem. Soc. Trans. 2008, 16, 255. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khosravi, A.; Addou, R.; Catalano, M.; Kim, J.; Wallace, R.M. High-κ Dielectric on ReS2: In-Situ Thermal Versus Plasma-Enhanced Atomic Layer Deposition of Al2O3. Materials 2019, 12, 1056. https://doi.org/10.3390/ma12071056
Khosravi A, Addou R, Catalano M, Kim J, Wallace RM. High-κ Dielectric on ReS2: In-Situ Thermal Versus Plasma-Enhanced Atomic Layer Deposition of Al2O3. Materials. 2019; 12(7):1056. https://doi.org/10.3390/ma12071056
Chicago/Turabian StyleKhosravi, Ava, Rafik Addou, Massimo Catalano, Jiyoung Kim, and Robert M. Wallace. 2019. "High-κ Dielectric on ReS2: In-Situ Thermal Versus Plasma-Enhanced Atomic Layer Deposition of Al2O3" Materials 12, no. 7: 1056. https://doi.org/10.3390/ma12071056
APA StyleKhosravi, A., Addou, R., Catalano, M., Kim, J., & Wallace, R. M. (2019). High-κ Dielectric on ReS2: In-Situ Thermal Versus Plasma-Enhanced Atomic Layer Deposition of Al2O3. Materials, 12(7), 1056. https://doi.org/10.3390/ma12071056