Electron Paramagnetic Resonance Study on Oxygen Vacancies and Site Occupations in Mg-Doped BaTiO3 Ceramics
Abstract
:1. Introduction
2. Methods
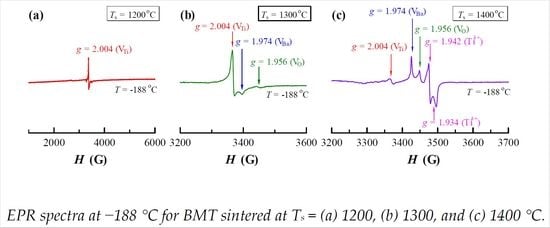
3. Results
4. Discussion
4.1. Site Occupation of Mg2+ in BM1T at Different Sintering Temperatures
4.2. Oxygen Vacancies and Dielectric Loss
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Liu, H.; Hao, H.; Zhang, S.; Sun, Y.; Zhang, W.; Zhang, L.; Cao, M. Microstructure effect on dielectric Properties of MgO-doped BaTiO3–BiYO3 ceramics. Ceram. Int. 2015, 41, 7489–7495. [Google Scholar]
- Lin, Y.T.; Ou, S.F.; Lin, M.H.; Song, Y.R. Effect of MgO addition on the microstructure and dielectric properties of BaTiO3 ceramics. Ceram. Int. 2018, 44, 3531–3535. [Google Scholar]
- Li, J.-H.; Wang, S.-F.; Hsu, Y.-F.; Chung, T.-F.; Yang, J.-R. Effects of Sc2O3 and MgO additions on the dielectric properties of BaTiO3-based X8R materials. J. Alloy. Compd. 2018, 768, 122–129. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Zhou, X.; Li, B.; Chen, Z. Effect of sintering atmospheres on the microstructure and dielectric properties of Yb/Mg co-doped BaTiO3 ceramics. Mater. Lett. 2005, 59, 2457–2460. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, K.J.; Yoon, Y.J.; Hong, M.H.; Hong, J.O.; Hur, K.H. Role of Yttium and magnesium in the formation of core-shell structure of BaTiO3 grains in MLCC. J. Eur. Ceram. Soc. 2008, 28, 1213–1219. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, W.N.; Huang, C.Y. Effect of MgO and Y2O3 doping on the formation of core-shell structure in BaTiO3 ceramics. J. Am. Ceram. Soc. 2013, 96, 2570–2576. [Google Scholar] [CrossRef]
- Nagai, T.; Iijima, K.; Hwang, H.J.; Sando, M.; Sekino, T.; Niihara, K. Effect of MgO doping on the phase transformations of BaTiO3. J. Am. Ceram. Soc. 2000, 83, 107–112. [Google Scholar] [CrossRef]
- Hagemann, H.J.; Hennings, D. Reversible weight change of acceptor-doped BaTiO3. J. Am. Ceram. Soc. 1981, 64, 590–594. [Google Scholar]
- Lu, D.; Ji, L.; Liu, J. Aging-resistant behavior and room-temperature electron spin resonance of Nd3+ in singly and doubly doped BaTiO3 ceramics associated with preservation history. Materials 2019, 12, 451. [Google Scholar] [CrossRef]
- Cha, S.H.; Han, Y.H. Effects of Mn doping on dielectric properties of Mg-doped BaTiO3. J. Appl. Phys. 2006, 100, 104102. [Google Scholar]
- Dong, M.; Miao, H.; Tan, G.; Pu, Y. Effects of Mg-doping on the microstructure and properties of BaTiO3 ceramics prepared by hydrothermal method. J. Electroceram. 2008, 21, 573–576. [Google Scholar] [CrossRef]
- Yoon, S.H.; Randall, C.A.; Hur, K.H. Effects of acceptor concentration on the bulk electrical conduction in acceptor (Mg)-doped BaTiO3. J. Appl. Phys. 2010, 107, 103721. [Google Scholar] [CrossRef]
- Kishi, H.; Mizuno, Y.; Chazono, H. Base-metal electrode-multilayer ceramic capacitors: past, present and future perspectives. Jpn. J. Appl. Phys. 2003, 42, 1–15. [Google Scholar] [CrossRef]
- Lu, D.; Cui, S. Defects characterisation of Dy-doped BaTiO3 ceramics via electron paramagnetic resonance. J. Eur. Ceram. Soc. 2014, 34, 2217–2227. [Google Scholar] [CrossRef]
- Cui, S.; Lu, D. Study of the solubility of La3+-Dy3+ defect complexes and dielectric properties of (Ba1−xLax)(Ti1−xDyx)O3 ceramics. Ceram. Int. 2015, 41, 2301–2308. [Google Scholar] [CrossRef]
- Lu, D.; Yin, S.; Cui, S. A fine-grained and low-loss X8R (Ba1–xDyx)(Ti1–x/2Cax/2)O3 ceramic. J. Alloy. Compd. 2018, 762, 282–288. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Bernard, J.; Houivet, D.; El Fallah, J.; Haussonne, J.M. MgTiO3 for Cu base metal multilayer ceramic capacitors. J. Eur. Ceram. Soc. 2004, 24, 1877–1881. [Google Scholar] [CrossRef]
- Lu, D.; Yuan, L.; Liang, W.; Zhu, Z. Characterization of oxygen vacancy defects in Ba1−xCaxTiO3 insulating ceramics using electron paramagnetic resonance technique. Jpn. J. Appl. Phys. 2016, 55, 011501. [Google Scholar] [CrossRef]
- Jayanthi, S.; Kutty, T.R.N. Dielectric properties of barium magnesiotitanate ceramics, BaMg6Ti6O19. Mater. Lett. 2006, 60, 796–800. [Google Scholar] [CrossRef]
- Dunbar, T.D.; Warren, W.L.; Tuttle, B.A.; Randall, C.A.; Tsur, T. Electron paramagnetic resonance investigations of lanthanide-doped barium titanate: dopant site occupancy. J. Phys. Chem. B 2004, 108, 908–917. [Google Scholar] [CrossRef]
- Kolodiazhnyi, T.; Petric, A. Analysis of point defects in polycrystalline BaTiO3 by electron paramagnetic resonance. J. Phys. Chem. Solids 2003, 64, 953–960. [Google Scholar] [CrossRef]
- Lu, D.; Liu, T. Dielectric properties and defect chemistry of (Ba1−xLax)(Ti1−xLux)O3 ceramics. J. Alloy. Compd. 2017, 698, 967–976. [Google Scholar] [CrossRef]
- Lu, D.; Sun, X. A novel electron paramagnetic resonance phenomenon in a barium stronium titanate ceramic. Adv. Mater. Res. 2011, 295–297, 1050–1054. [Google Scholar] [CrossRef]
- El Ghadraoui, O.E.; Zouhairi, M.; Abdi, F.; Lamcharfi, T.; Bali, H.; El Ghadraoui, E.; Aillerie, M. Preparation and physico-chemical study of Mg doped BaTiO3 (Ba1−xMgxTiO3) prepared by the sol-gel method. Int. J. Curr. Res. 2016, 8, 42815–42820. [Google Scholar]
- Kirianov, A.; Ozaki, N.; Ohsato, H.; Kohzu, N.; Kishi, H. Studies on the solid solution of Mn in BaTiO3. Jpn. J. Appl. Phys. 2001, 40, 5619–5623. [Google Scholar] [CrossRef]
- Dang, N.V.; Phan, T.-L.; Thanh, T.D.; Lam, V.D.; Hong, L.V. Structural phase separation and optical and magnetic properties of Ba(Ti1−xMnx)O3 multiferroics. J. Appl. Phys. 2012, 111, 113913. [Google Scholar] [CrossRef]
- Langhammer, H.T.; Muller, T.; Felgner, K.H.; Abicht, H.P. Crystal structure and related properties of manganese-doped barium titanate ceramics. J. Am. Ceram. Soc. 2000, 83, 605–611. [Google Scholar] [CrossRef]





| Ion | CN | r (Å) |
|---|---|---|
| Ba2+ | 12 | 1.61 |
| Ti4+ | 6 | 0.605 |
| Ti3+ | 6 | 0.67 |
| Mg2+ | 12 | 1.23 |
| Mg2+ | 6 | 0.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Zheng, Y.; Yuan, L. Electron Paramagnetic Resonance Study on Oxygen Vacancies and Site Occupations in Mg-Doped BaTiO3 Ceramics. Materials 2019, 12, 1525. https://doi.org/10.3390/ma12091525
Lu D, Zheng Y, Yuan L. Electron Paramagnetic Resonance Study on Oxygen Vacancies and Site Occupations in Mg-Doped BaTiO3 Ceramics. Materials. 2019; 12(9):1525. https://doi.org/10.3390/ma12091525
Chicago/Turabian StyleLu, Dayong, Yongshun Zheng, and Longfei Yuan. 2019. "Electron Paramagnetic Resonance Study on Oxygen Vacancies and Site Occupations in Mg-Doped BaTiO3 Ceramics" Materials 12, no. 9: 1525. https://doi.org/10.3390/ma12091525
APA StyleLu, D., Zheng, Y., & Yuan, L. (2019). Electron Paramagnetic Resonance Study on Oxygen Vacancies and Site Occupations in Mg-Doped BaTiO3 Ceramics. Materials, 12(9), 1525. https://doi.org/10.3390/ma12091525