Figure 1.
Effect of different reaction parameters on % yield of PANI (a) Amount of monomer, (b) Amount of Oxidant, (c) Amount of dopant in mmol, (d) Amount of solvent in mL, and (e) UV/Visible spectrum filtrate from product washing indicating the presence of oligomers.
Figure 1.
Effect of different reaction parameters on % yield of PANI (a) Amount of monomer, (b) Amount of Oxidant, (c) Amount of dopant in mmol, (d) Amount of solvent in mL, and (e) UV/Visible spectrum filtrate from product washing indicating the presence of oligomers.
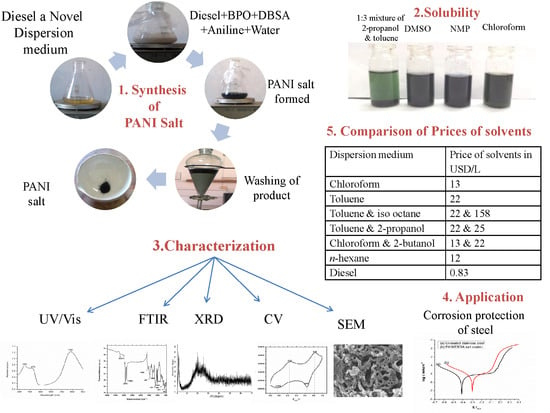
Figure 2.
Solutions of PANI salt in different solvents as indicated.
Figure 2.
Solutions of PANI salt in different solvents as indicated.
Figure 3.
UV/Visible Spectra of different PANI samples indicated in
Table 1,
Table 2,
Table 3 and
Table 4. (
a) Monomer optimized samples (
b) Oxidant optimized samples (
c) DBSA optimized samples (
d) Solvents optimized samples.
Figure 3.
UV/Visible Spectra of different PANI samples indicated in
Table 1,
Table 2,
Table 3 and
Table 4. (
a) Monomer optimized samples (
b) Oxidant optimized samples (
c) DBSA optimized samples (
d) Solvents optimized samples.
Figure 4.
UV/Visible spectra of PANI A4, PANI D2, PANI B9 and PANI S3.
Figure 4.
UV/Visible spectra of PANI A4, PANI D2, PANI B9 and PANI S3.
Figure 5.
UV/Visible spectrum of PANI salt.
Figure 5.
UV/Visible spectrum of PANI salt.
Figure 6.
FTIR spectrum of different PANI samples as indicated.
Figure 6.
FTIR spectrum of different PANI samples as indicated.
Figure 7.
FTIR spectrum of PANI salt.
Figure 7.
FTIR spectrum of PANI salt.
Figure 8.
X-ray diffractograms of (a) PANI A4 (b) PANI B9 (c) PANI D2 (d) PANI S3.
Figure 8.
X-ray diffractograms of (a) PANI A4 (b) PANI B9 (c) PANI D2 (d) PANI S3.
Figure 9.
X-ray diffractogram of PANI salt.
Figure 9.
X-ray diffractogram of PANI salt.
Figure 10.
CV of synthesized PANI samples coated on a gold sheet electrode, in 0.5 M H2SO4 at a scan rate of 50 mV/s (a) PANI A4 (b) PANI B9 (c) PANI D2 (d) PANI S3.
Figure 10.
CV of synthesized PANI samples coated on a gold sheet electrode, in 0.5 M H2SO4 at a scan rate of 50 mV/s (a) PANI A4 (b) PANI B9 (c) PANI D2 (d) PANI S3.
Figure 11.
Cyclic voltammogram of PANI salt coated on a gold sheet electrode, in 0.5 M H2SO4 at a scan rate of 50 mV/s.
Figure 11.
Cyclic voltammogram of PANI salt coated on a gold sheet electrode, in 0.5 M H2SO4 at a scan rate of 50 mV/s.
Figure 12.
(a) Tafel plot of uncoated and different PANI coated stainless steel with different samples, as indicated. (b) Tafel plot of PANI coated via different no of drops on steel.
Figure 12.
(a) Tafel plot of uncoated and different PANI coated stainless steel with different samples, as indicated. (b) Tafel plot of PANI coated via different no of drops on steel.
Figure 13.
Tafel plot of (a) uncoated and, (b) PANI coated stainless steel.
Figure 13.
Tafel plot of (a) uncoated and, (b) PANI coated stainless steel.
Figure 14.
Scanning electron micrograph (a) PANI A4 (b) PANI B9 (c) PANI D2 (d) and PANI S3.
Figure 14.
Scanning electron micrograph (a) PANI A4 (b) PANI B9 (c) PANI D2 (d) and PANI S3.
Figure 15.
Scanning electron micrograph of PANI salt.
Figure 15.
Scanning electron micrograph of PANI salt.
Figure 16.
Thermograms of different (a) PANI A4 (b) PANI B9 (c) PANI D2 and (d) PANI S3.
Figure 16.
Thermograms of different (a) PANI A4 (b) PANI B9 (c) PANI D2 and (d) PANI S3.
Figure 17.
TGA curve of PANI salt.
Figure 17.
TGA curve of PANI salt.
Table 1.
Price of solvents in USD/L (2018/2019) and its effect on product properties.
Table 1.
Price of solvents in USD/L (2018/2019) and its effect on product properties.
| Dispersion Medium | Price of Solvents in USD/L | Maximum % Yield | Thermal Stability
(°C) | Solubility | Reference |
|---|
| Chloroform | 13 | 52.4 | 480 | NMP, DMSO, dimethylformamide (DMF) | [6] |
| Toluene | 22 | - | - | Toluene, Xylene | [11] |
| Toluene & iso octane | 22 & 158 | 108–194 | 320–499 | DMSO | [16] |
| Toluene & 2-propanol | 22 & 25 | 61 | - | Chlorofrom, 2:1 mixture of toluene & 2-propanol, NMP, Dichloromethane. | [7] |
| Chloroform & 2-butanol | 13 & 22 | 25–30 | 500 | 2:1 mixture of toluene + 2-propanol, chloroform, DMSO & DMF. | [10] |
| n-hexane | 12 | 81.97 | 320 | - | [17] |
| Diesel | 0.83 | 87.6 | 417 | Chloroform, NMP, DMSO, 1:3 Mixture of toluene & 2-propanol | Present work |
Table 2.
Effect of amount of monomer (aniline) on percent yield of PANI.
Table 2.
Effect of amount of monomer (aniline) on percent yield of PANI.
| SerialNo | mmol of Aniline | Weight of the PANI (g) | % yield | Sample Code |
|---|
| 1 | 0.55 | 0.000 | 0.00 | PANI A1 |
| 2 | 1.10 | 0.004 | 4.00 | PANI A2 |
| 3 | 1.60 | 0.023 | 15.64 | PANI A3 |
| 4 | 2.20 | 0.065 | 31.90 | PANI A4 |
| 5 | 2.74 | 0.060 | 24.40 | PANI A5 |
| 6 | 3.30 | 0.071 | 24.10 | PANI A6 |
| 7 | 3.83 | 0.054 | 15.12 | PANI A7 |
Table 3.
Effect of amount of oxidant (Benzoyl peroxide) on percent yield PANI.
Table 3.
Effect of amount of oxidant (Benzoyl peroxide) on percent yield PANI.
| Serial No | mmol of Oxidant | Weight of PANI (g) | % yield | Sample Code |
|---|
| 1 | 0.21 | 0.000 | 0.00 | PANI B1 |
| 2 | 0.41 | 0.001 | 0.49 | PANI B2 |
| 3 | 0.83 | 0.005 | 2.45 | PANI B3 |
| 4 | 1.03 | 0.008 | 3.92 | PANI B4 |
| 5 | 1.24 | 0.026 | 12.74 | PANI B5 |
| 6 | 1.65 | 0.065 | 31.86 | PANI B6 |
| 7 | 2.06 | 0.080 | 40.8 | PANI B7 |
| 8 | 2.48 | 0.124 | 60.7 | PANI B8 |
| 9 | 2.90 | 0.178 | 87.6 | PANI B9 |
| 10 | 3.30 | 0.123 | 60.4 | PANIB10 |
Table 4.
Effect of amount of dopant (DBSA) on percent yield of PANI.
Table 4.
Effect of amount of dopant (DBSA) on percent yield of PANI.
| Serial No | mmoles of DBSA | Weight of PANI (g) | % yield | Sample Code |
|---|
| 1 | 0.91 | 0.065 | 31.86 | PANI D1 |
| 2 | 1.52 | 0.116 | 56.86 | PANI D2 |
| 3 | 2.13 | 0.112 | 54.90 | PANI D3 |
| 4 | 3.04 | 0.096 | 47.05 | PANI D4 |
| 5 | 3.65 | 0.045 | 22.05 | PANI D5 |
| 6 | 4.56 | 0.084 | 41.17 | PANI D6 |
Table 5.
Effect of amount of solvents (diesel/water) on percent yield of PANI.
Table 5.
Effect of amount of solvents (diesel/water) on percent yield of PANI.
| Serial No | Amount of Organic Solvent (mL) | Amount of Water (mL) | Weight of PANI (g) | % Yield | Sample Code |
|---|
| 1 | 50 | 10 | 0.026 | 12.574 | PANI S1 |
| 2 | 40 | 20 | 0.042 | 20.58 | PANI S2 |
| 3 | 30 | 30 | 0.043 | 21.07 | PANI S3 |
| 4 | 20 | 40 | 0.030 | 14.70 | PANI S4 |
| 5 | 10 | 50 | 0.036 | 17.64 | PANI S5 |
Table 6.
The extent of doping of DBSA into PANI Chain.
Table 6.
The extent of doping of DBSA into PANI Chain.
| Serial No | Sample | E/B |
|---|
| 01 | PANI A4 | 2.21 |
| 02 | PANI D2 | 2.25 |
| 03 | PANI B9 | 2.14 |
| 04 | PANI S3 | 2.22 |
Table 7.
Peak assignments in the FTIR spectra of PANI salt.
Table 7.
Peak assignments in the FTIR spectra of PANI salt.
| Serial No | Peak Positions (cm−1) | Peak Assignment |
|---|
| 01 | 503 and 573 | –SO3H and SO3−1 of DBSA |
| 02 | 798 | Out of plane C–H bending vibration |
| 03 | 1002 | –SO3H of DBSA |
| 04 | 1122 | In-plane bending vibration of C–H |
| 05 | 1298 | C–N stretching of benzenoid ring |
| 06 | 1467 | Benzenoid ring |
| 07 | 1565 | Quinoid ring C–N strecting |
| 08 | 2853 and 2916 | C–H stretching vibrations of aromatic aniline ring |
| 09 | 3232 | Symmetric and asymmetric stretching of NH2 and NH |
Table 8.
Values of icorr (µA), Ecorr (mV), βA (V/decade), βC (V/decade) and CR (mm y−1) for different samples of PANI salt.
Table 8.
Values of icorr (µA), Ecorr (mV), βA (V/decade), βC (V/decade) and CR (mm y−1) for different samples of PANI salt.
| Material | icorr (µA) | Ecorr (mV) | βA (V/decade) | βC (V/decade) | CR (mm y−1) |
|---|
| Uncoated steel | 10.70 | −467.0 | 0.5304 | 0.2708 | 4.906 |
| PANI A4 | 7.050 | −231.0 | 0.1243 | 0.1818 | 3.223 |
| PANI B9 | 3.450 | −184.0 | 0.959 | 0.2586 | 1.575 |
| PANI D2 | 4.870 | −400.0 | 0.272 | 0.221 | 2.226 |
| PANI S3 | 4.380 | 320.0 | 0.2865 | 0.1875 | 2.000 |
| PANI salt | 0.859 | −295.0 | 0.1086 | 0.1194 | 0.3927 |