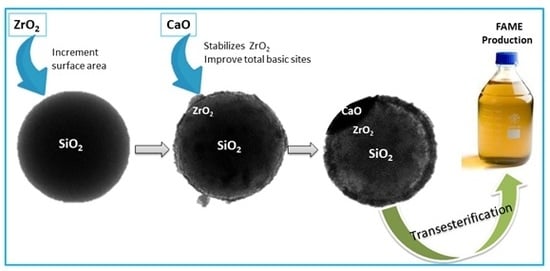
The Effect of the ZrO2 Loading in SiO2@ZrO2-CaO Catalysts for Transesterification Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SiO2 Spheres
2.1.1. Synthesis of SiO2@ZrO2
2.1.2. Synthesis of SiO2@ZrO2-CaO
2.2. Catalytic Tests and Product Analysis
2.3. Catalyst Recovery
2.4. Core–Shell Characterization
3. Results
3.1. Reproducibility and Thermal Stability of the SiO2 Sphere
3.2. Textural Characterization for Core–Shell
3.2.1. BET Surface Area
3.2.2. TEM Analysis
3.3. SEM-EDS Analyses
3.4. FTIR Analysis
3.5. X-Ray Powder Diffraction
3.6. Raman Spectroscopy
3.7. Carbon Dioxide Temperature-Programmed Desorption
3.8. Reusability SiO2@ZrO2-CaO Core–Shell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reyna-Villanueva, L.R.M.; Dias, J.; Medellín-Castillo, N.A.; Ocampo-Pérez, R.; Martínez-Rosales, J.M.; Peñaflor-Galindo, T.; Fuentes, G.A. Biodiesel production using layered double hidroxides and derived mixed oxides: The role of the synthesis conditions and the catalysts properties on biodiesel conversion. Fuel 2019, 251, 285–292. [Google Scholar] [CrossRef]
- Jindapon, W.; Ngamcharussrivichai, C. Heterogeneously catalyzed transesterification of palm oil with methanol to produce biodiesel over calcined dolomite: The role of magnesium oxide. Energy Convers. Manag. 2018, 171, 1311–1321. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, V.; Baranwal, A.; Chandra, P.; Pandey, L.M. Engineered nanoporous materials mediated heterogeneous catalysts and their implications in biodiesel production. Mater. Sci. Energy Technol. 2018, 1, 11–21. [Google Scholar] [CrossRef]
- Esipovich, A.L.; Rogozhin, A.E.; Belousov, A.S.; Kanakov, E.A.; Otopkova, K.V.; Danov, S.M. Liquid–liquid equilibrium in the systems FAMEs+vegetable oil+methyl alcohol and FAMEs+glycerol+methyl alcohol. Fuel 2018, 217, 31–37. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghuimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Sun, H.; Ding, Y.; Duan, J.; Zhang, Q.; Wang, Z.; Lou, H.; Zheng, X. Transesterification of sunflower oil to biodiesel on ZrO2 supported La2O3 catalyst. Bioresour. Technol. 2010, 101, 953–958. [Google Scholar] [CrossRef]
- Onukwuli, D.O.; Emembolu, L.N.; Ude, C.N.; Aliozo, S.O.; Menkiti, M.C. Optimization of biodiesel production from refined cotton seed oil and its characterization. Egypt. J. Pet. 2017, 26, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Da Silva César, A.; Conejero, M.A.; Barros Ribeiro, E.C.; Batalha, M.O. Competitiveness analysis of “social soybeans” in biodiesel production in Brazil. Renew. Energy 2019, 133, 1147–1157. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Haapaniemi, E.; Sillanpää, M. Nano-magnetic potassium impregnated ceria as catalyst for the biodiesel production. Renew. Energy 2019, 139, 1428–1436. [Google Scholar] [CrossRef]
- Alsharifi, M.; Znad, H.; Hena, S.; Ang, M. Biodiesel production from canola oil using novel Li/TiO2 as a heterogeneous catalyst prepared via impregnation method. Renew. Energy 2017, 114, 1077–1089. [Google Scholar] [CrossRef]
- Salinas, D.; Sepúlveda, C.; Escalona, N.; Gfierro, J.L.; Pecchi, G. Sol–gel La2O3–ZrO2 mixed oxide catalysts for biodiesel production. J. Energy Chem. 2018, 27, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Ntaribi, T.; Paul, D.I. Status of Jatropha plants farming for biodiesel production in Rwanda. Energy Sustain. Dev. 2018, 47, 133–142. [Google Scholar] [CrossRef]
- Castro Gonzáles, N.F. International experiences with the cultivation of Jatropha curcas for biodiesel production. Energy 2016, 112, 1245–1258. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crop. Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Jain, S. The production of biodiesel using Karanja (Pongamia pinnata) and Jatropha (Jatropha curcas) Oil. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 397–408. [Google Scholar]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M.; Kale, P.; Geremew, B.; Adeloju, S.B.; Nizami, M.; Ayoub, M. Optimization of process variables for biodiesel production by transesterification of flaxseed oil and produced biodiesel characterizations. Renew. Energy 2019, 139, 1272–1280. [Google Scholar] [CrossRef]
- Shomal, R.; Hisham, H.; Mlhem, A.; Hassan, R.; Al-Zuhair, S. Simultaneous extraction–reaction process for biodiesel production from microalgae. Energy Rep. 2019, 5, 37–40. [Google Scholar] [CrossRef]
- De Luna, M.D.G.; Doliente, L.M.T.; Ido, A.L.; Chung, T.-W. In situ transesterification of Chlorella sp. microalgae using LiOH-pumice catalyst. J. Environ. Chem. Eng. 2017, 5, 2830–2835. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]
- Akubude, V.C.; Nwaigwe, K.N.; Dintwa, E. Production of biodiesel from microalgae via nanocatalyzed transesterification process: A review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Chao, C.-Y.; Tsai, H.-W.; Pan, K.-L.; Hsieh, C.-W. On the microexplosion mechanisms of burning droplets blended with biodiesel and alcohol. Combust. Flame 2019, 205, 397–406. [Google Scholar] [CrossRef]
- Erdiwansyah Mamat, R.; Sani, M.S.M.; Sudhakar, K.; Kadarohman, A.; Sardjono, R.E. An overview of Higher alcohol and biodiesel as alternative fuels in engines. Energy Rep. 2019, 5, 467–479. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, Z.; Chen, Y.; Zhang, P.; Duan, S.; Liu, X.; Mao, Z. Preparation of Biodiesel Catalyzed by Solid Super Base of Calcium Oxide and Its Refining Process. Chin. J. Catal. 2006, 27, 391–396. [Google Scholar] [CrossRef]
- Ho, W.W.S.; Ng, H.K.; Gan, S.; Tan, S.H. Evaluation of palm oil mill fly ash supported calcium oxide as a heterogeneous base catalyst in biodiesel synthesis from crude palm oil. Energy Convers. Manag. 2014, 88, 1167–1178. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Catarino, M.; Martins, S.; Soares Dias, A.P.; Costa Pereira, M.F.; Gomes, J. Calcium diglyceroxide as a catalyst for biodiesel production. J. Environ. Chem. Eng. 2019, 7, 103099. [Google Scholar] [CrossRef]
- Devaraj, K.; Veerasamy, M.; Aathika, S.; Mani, Y.; Thanarasu, A.; Dhanasekaran, A.; Subramanian, S. Study on effectiveness of activated calcium oxide in pilot plant biodiesel production. J. Clean. Prod. 2019, 225, 18–26. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, D.-Y.; Chen, B.-H. Metasilicate-based catalyst prepared from natural diatomaceous earth for biodiesel production. Renew. Energy 2019, 138, 1042–1050. [Google Scholar] [CrossRef]
- Corro, G.; Flores, A.; Pacheco-Aguirre, F.; Pal, U.; Bañuelos, F.; Ramirez, A.; Zehe, A. Biodiesel and fossil-fuel diesel soot oxidation activities of Ag/CeO2 catalyst. Fuel 2019, 250, 17–26. [Google Scholar] [CrossRef]
- Banković–Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of nano CaO–based catalysts in biodiesel synthesis. Renew. Sustain. Energy Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.B.; Cornejo, D.R.; Costa, A.C.F.M. Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel 2017, 191, 463–471. [Google Scholar] [CrossRef]
- Harsha Hebbar, H.R.; Math, M.C.; Yatish, K.V. Optimization and kinetic study of CaO nano-particles catalyzed biodiesel production from Bombax ceiba oil. Energy 2018, 143, 25–34. [Google Scholar] [CrossRef]
- Li, G.; Tang, Z. Noble metal nanoparticle@metal oxide core/yolk–shell nanostructures as catalysts: recent progress and perspective. Nanoscale 2014, 6, 3995–4011. [Google Scholar] [CrossRef] [PubMed]
- Gazzoli, D.; Mattei, G.; Valigi, M. Raman and X-ray investigations of the incorporation of Ca2+ and Cd2+ in the ZrO2 structure. J. Raman Spectrosc. 2007, 38, 824–831. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Muhtaseb Aa Myint, M.T.Z.; Al-Hinai, M.; Al-Haj, L.; Baawain, M.; Al-Abri, M.; Kumar, G.; Atabani, A.E. Biodiesel production by valorizing waste Phoenix dactylifera L. Kernel oil in the presence of synthesized heterogeneous metallic oxide catalyst (Mn@MgO-ZrO2). Energy Convers. Manag. 2018, 155, 128–137. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzón, A. Methane reforming with CO2 over Ni/ZrO2–CeO2 catalysts prepared by sol–gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, C.; Yuan, K.; Gan, X.; Feng, C.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. Characterization and adsorption mechanism of ZrO2 mesoporous fibers for health-hazardous fluoride removal. J. Hazard. Mater. 2018, 346, 82–92. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, B.; Zhou, H.; Feng, C.; Wang, X.; Yuan, K.; Gan, X.; Zhu, L.; Zhang, G.; Xu, D. Mesoporous ZrO2 fibers with enhanced surface area and the application as recyclable absorbent. Appl. Surf. Sci. 2017, 399, 288–297. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Structure and Thermomechanical Properties of Partially Stabilized Zirconia in the CaO-ZrO2 System. In Sintering Key Papers; Sōmiya, S., Moriyoshi, Y., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 259–273. [Google Scholar]
- Zhang, M.; Gao, L.; Kang, J.; Pu, J.; Peng, J.; Omran, M.; Chen, G. Stability optimisation of CaO-doped partially stabilised zirconia by microwave sintering. Ceram. Int. 2019, 45, 23278–23282. [Google Scholar] [CrossRef]
- Albuquerque, E.; Borges, L.; Fraga, M.; Sievers, C. Relationship between acid-base properties and the activity of ZrO2 catalysts for the Cannizzaro reaction of pyruvaldehyde to lactic acid. ChemCatChem 2017, 9. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Farhadi, K.; Mansourpanah, Y.; Nikbakht, A.M.; Molaei, R.; Forough, M. Application of CaO-based/Au nanoparticles as heterogeneous nanocatalysts in biodiesel production. Fuel 2016, 164, 119–127. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Zhang, Y.; Zhou, S.; Shi, J.; Kong, J.; Zhang, S. Well-crystallized mesoporous TiO2 shells for enhanced photocatalytic activity: prepared by carbon coating and silica-protected calcination. Dalton Trans. 2013, 42, 5004–5012. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Chang, S.M.; Kim, S.; Kim, K.-S.; Kim, J.; Kim, W.-S. Design of SiO2/ZrO2 core–shell particles using the sol–gel process. Ceram. Int. 2009, 35, 1243–1247. [Google Scholar] [CrossRef]
- Li, S.; Cai, J.; Wu, X.; Liu, B.; Chen, Q.; Li, Y.; Zheng, F. TiO2@Pt@CeO2 nanocomposite as a bifunctional catalyst for enhancing photo-reduction of Cr (VI) and photo-oxidation of benzyl alcohol. J. Hazard. Mater. 2018, 346, 52–61. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; p. 56. [Google Scholar]
- Dubey, R.S.; Rajesh, Y.B.R.D.; More, M.A. Synthesis and Characterization of SiO2 Nanoparticles via Sol-gel Method for Industrial Applications. Mater. Today Proc. 2015, 2, 3575–3579. [Google Scholar] [CrossRef]
- Hu, L.; Li, N.; Dai, X.; Guo, Y.; Jiang, Y.; He, A.; Xu, J. Highly efficient production of 2,5-dihydroxymethylfuran from biomass-derived 5-hydroxymethylfurfural over an amorphous and mesoporous zirconium phosphonate catalyst. J. Energy Chem. 2019, 37, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.S.; Verma, T.N. Taguchi design approach for extraction of methyl ester from waste cooking oil using synthesized CaO as heterogeneous catalyst: Response surface methodology optimization. Energy Convers. Manag. 2019, 182, 383–397. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Hadadian, M. Effect of calcination temperature on structural, vibrational, optical, and rheological properties of zirconia nanoparticles. Ceram. Int. 2012, 38, 1771–1777. [Google Scholar] [CrossRef]
- Hong, S.-J.; Han, J.-I. Eco-friendly synthesis of SiO2 nanoparticles with high purity for digital printing. Thin Solid Film. 2010, 518, 6634–6637. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhou, Y.; Sun, Y.; Chen, J.; Wang, Z. Preparation and characterization of SiO2/ZrO2/Ag multicoated microspheres. Appl. Surf. Sci. 2008, 254, 1942–1946. [Google Scholar] [CrossRef]
- Borah, M.J.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Promarak, V. Biodiesel production from palm oil using hydrated lime-derived CaO as a low-cost basic heterogeneous catalyst. Energy Convers. Manag. 2016, 108, 459–467. [Google Scholar] [CrossRef]
- Alessi, A.; Agnello, S.; Buscarino, G.; Gelardi, F.M. Structural properties of core and surface of silica nanoparticles investigated by Raman spectroscopy. J. Raman Spectrosc. 2013, 44, 810–816. [Google Scholar] [CrossRef]
- Alessi, A.; Agnello, S.; Buscarino, G.; Gelardi, F.M. Raman and IR investigation of silica nanoparticles structure. J. Non-Cryst. Solids 2013, 362, 20–24. [Google Scholar] [CrossRef]
- Kucharczyk, S.; Sitarz, M.; Zajac, M.; Deja, J. The effect of CaO/SiO2 molar ratio of CaO-Al2O3-SiO2 glasses on their structure and reactivity in alkali activated system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 163–171. [Google Scholar] [CrossRef]
- Salinas, D.; Sepulveda, C.; Escalona, N.; Pecchi, G. Environmentally friendly heterogeneous sol–gel La2O3–Al2O3 mixed oxides for transesterification reaction. Chem. Pap. 2018, 72, 2353–2362. [Google Scholar] [CrossRef]













| Catalyst | BET Surface Area (m2 g−1) 700 °C | Pore Diameter (nm) 700 °C | Basic Properties (μmol g−1) |
|---|---|---|---|
| SiO2 spheres | 15 | 5.0 | n.d |
| SiO2@ZrO2 0.04 M | 45 | 4.0 | 28 |
| SiO2@ZrO2 0.06 M | 23 | 3.7 | 69 |
| SiO2@ZrO2 0.08 M | 36 | 3.4 | 46 |
| SiO2@ZrO2 0.04 M–CaO | 13 | n.d | 273 |
| SiO2@ZrO2 0.06 M–CaO | 19 | 6.4 | 306 |
| SiO2@ZrO2 0.08 M–CaO | 15 | 6.1 | 454 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, D.; Guerrero, S.; Campos, C.H.; Bustamante, T.M.; Pecchi, G. The Effect of the ZrO2 Loading in SiO2@ZrO2-CaO Catalysts for Transesterification Reaction. Materials 2020, 13, 221. https://doi.org/10.3390/ma13010221
Salinas D, Guerrero S, Campos CH, Bustamante TM, Pecchi G. The Effect of the ZrO2 Loading in SiO2@ZrO2-CaO Catalysts for Transesterification Reaction. Materials. 2020; 13(1):221. https://doi.org/10.3390/ma13010221
Chicago/Turabian StyleSalinas, Daniela, Sichem Guerrero, Cristian H. Campos, Tatiana M. Bustamante, and Gina Pecchi. 2020. "The Effect of the ZrO2 Loading in SiO2@ZrO2-CaO Catalysts for Transesterification Reaction" Materials 13, no. 1: 221. https://doi.org/10.3390/ma13010221
APA StyleSalinas, D., Guerrero, S., Campos, C. H., Bustamante, T. M., & Pecchi, G. (2020). The Effect of the ZrO2 Loading in SiO2@ZrO2-CaO Catalysts for Transesterification Reaction. Materials, 13(1), 221. https://doi.org/10.3390/ma13010221