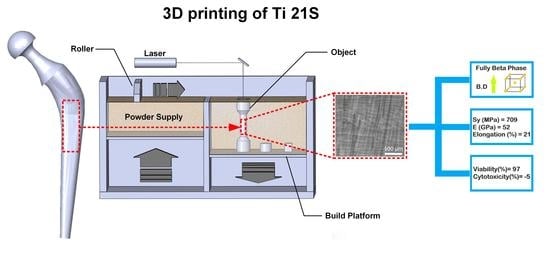
A 3D-Printed Ultra-Low Young’s Modulus β-Ti Alloy for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Microstructure
- Tliq = liquidus temperature;
- Tsol = solidus temperature;
- D = solute diffusivity in the liquid;
- v = solidification speed.
3.2. Phase Constitution and Texture
3.3. Mechanical Properties
3.4. In Vitro Cytotoxicity
4. Conclusions
- A fully metastable β structure could be obtained, even despite the rapid solidification;
- Columnar grains formed along the building direction led to texture in <100> orientation. Inside melting pools, the solidification mechanism changed from planar to cellular owing to the establishment of strong constitutional undercooling, caused by the wide alloy freezing range.
- Very interesting mechanical properties could be measured in the as-built state, without any post heat treatment; the Young’s modulus is one of the lowest reported in literature for β-Ti alloys (52 GPa), the mechanical strength is slightly lower than that of Ti–6Al–4V, in line with those of other β-Ti alloys. The high fracture elongation suggests the good strain accommodation capacity and the possibility of limiting distortions.
- Compression tests revealed that texture causes a limited variation (<20%) of the Young’s modulus along different directions.
- Viability results showed that experimental and reference samples had higher significant viability than CTR+, and no cytotoxicity was detected
Author Contributions
Funding
Conflicts of Interest
References
- Lütjering, G.; Williams, J.C. Titanium, 2nd ed.; Springer: Berlin, Germany, 2007; pp. 283–332. [Google Scholar]
- Collings, E. The Physical Metallurgy of Titanium Alloys, 1st ed.; Metals Park; American Society for Metals: Cleveland, OH, USA, 1984; pp. 23–240. [Google Scholar]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. 2010, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Relat. Res. 1992, 1, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—properties and fabrication: A review. J. Bioma. Ter. Tiss. Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Niinomi, M. Microstructures and mechanical properties of Ti–50 mass% Ta alloy for biomedical applications. J. Alloy Compd. 2008, 466, 535–542. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti–29Nb–13Ta–4.6 Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]
- Benedetti, M.; Fontanari, V.; Lütjering, G.; Albrecht, J. The effect of notch plasticity on the behaviour of fatigue cracks emanating from edge-notches in high-strength β-titanium alloys. Eng. Fract. Mech. 2008, 75, 169–187. [Google Scholar] [CrossRef]
- Peters, J.; Lütjering, G. Comparison of the fatigue and fracture of α + β and β titanium alloys. Metall. Mater. Trans. A 2001, 32, 2805–2818. [Google Scholar] [CrossRef]
- Veronesi, F.; Tschon, M.; Fini, M. Gene expression in osteolysis: Review on the identification of altered molecular pathways in preclinical and clinical studies. Int. J. Mol. Sci. 2017, 18, 499. [Google Scholar] [CrossRef] [Green Version]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [Green Version]
- Sundfeldt, M.; VCarlsson, L.; BJohansson, C.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Wooley, P.; Schwarz, E. Aseptic loosening. Gene Ther. 2004, 11, 402–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanmeensel, K.; Lietaert, K.; Vrancken, B.; Dadbakhsh, S.; Li, X.; Kruth, J.-P.; Krakhmalev, P.; Yadroitsev, I.; Van Humbeeck, J. Additively manufactured metals for medical applications. In Additive Manufacturing; Butterworth-Heinemann: Oxford, UK, 2018; pp. 261–309. [Google Scholar]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Ju, C.; Lin, J.C. Structure and properties of cast binary Ti–Mo alloys. Biomaterials 1999, 20, 2115–2122. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, S.; Zhang, F.; Kou, H.; Li, J. Effect of ω-assisted precipitation on β→ α transformation and tensile properties of Ti–15Mo–2.7 Nb–3Al–0.2Si alloy. Mater. Sci. Eng. A 2016, 654, 249–255. [Google Scholar] [CrossRef]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio. 2019, 20, 100024. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Tan, X.; Tan, Y.; Chow, C.; Tor, S.; Yeong, W. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef]
- Wang, Y.; Arabnejad, S.; Tanzer, M.; Pasini, D. Hip implant design with three-dimensional porous architecture of optimized graded density. J. Mech. Des. 2018, 140, 111406. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Han, C.; Choma, T.; Wei, Q.; Yan, C.; Song, B.; Shi, Y. Effect of Nb content on microstructure, property and in vitro apatite-forming capability of Ti-Nb alloys fabricated via selective laser melting. Mater. Des. 2017, 126, 268–277. [Google Scholar] [CrossRef]
- Zhuravleva, K.; Bönisch, M.; Prashanth, K.G.; Hempel, U.; Helth, A.; Gemming, T.; Calin, M.; Scudino, S.; Schultz, L.; Eckert, J. Production of porous β-Type Ti–40Nb alloy for biomedical applications: Comparison of selective laser melting and hot pressing. Materials 2013, 6, 5700–5712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yuan, T.; Li, R.; Tang, J.; Wang, M.; Mei, F. Microstructure and mechanical properties of selective laser melted biomaterial Ti-13Nb-13Zr compared to hot-forging. Mater. Sci. Eng. A 2018, 725, 329–340. [Google Scholar] [CrossRef]
- Fischer, M.; Joguet, D.; Robin, G.; Peltier, L.; Laheurte, P. In situ elaboration of a binary Ti–26Nb alloy by selective laser melting of elemental titanium and niobium mixed powders. Mater. Sci. Eng. C 2016, 62, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Schwab, H.; Prashanth, K.; Löber, L.; Kühn, U.; Eckert, J. Selective laser melting of Ti-45Nb alloy. Metals 2015, 5, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, X.; Zhang, L.C.; Sercombe, T. Processing and properties of topologically optimised biomedical Ti–24Nb–4Zr–8Sn scaffolds manufactured by selective laser melting. Mater. Sci. Eng. A 2015, 642, 268–278. [Google Scholar] [CrossRef]
- Luo, J.; Sun, J.; Huang, Y.; Zhang, J.; Zhang, Y.; Zhao, D.; Yan, M. Low-modulus biomedical Ti–30Nb–5Ta–3Zr additively manufactured by Selective Laser Melting and its biocompatibility. Mater. Sci. Eng. C 2018, 97, 275–284. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E. Selective laser melting of titanium alloy with 50 wt% tantalum: Microstructure and mechanical properties. J. Alloy Compd. 2016, 660, 461–470. [Google Scholar] [CrossRef]
- Yan, L.; Yuan, Y.; Ouyang, L.; Li, H.; Mirzasadeghi, A.; Li, L. Improved mechanical properties of the new Ti-15Ta-xZr alloys fabricated by selective laser melting for biomedical application. J. Alloy Compd. 2016, 688, 156–162. [Google Scholar] [CrossRef]
- Schwab, H.; Palm, F.; Kühn, U.; Eckert, J. Microstructure and mechanical properties of the near-beta titanium alloy Ti-5553 processed by selective laser melting. Mater. Des. 2016, 105, 75–80. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014, 68, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.; Li, Y.; Lin, X.; Feng, E.; Huang, W. Microstructure and tensile properties of Ti-Mo alloys manufactured via using laser powder bed fusion. J. Alloy Compd. 2019, 771, 877–884. [Google Scholar] [CrossRef]
- Kang, N.; Lin, X.; Coddet, C.; Wen, X.; Huang, W. Selective laser melting of low modulus Ti-Mo alloy: α/β heterogeneous conchoidal structure. Mater. Lett. 2020, 267, 127544. [Google Scholar] [CrossRef]
- Vilaro, T.; Colin, C.; Bartout, J.-D. As-fabricated and heat-treated microstructures of the Ti-6Al-4V alloy processed by selective laser melting. Metall. Mater. Trans. A 2011, 42, 3190–3199. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H. Phase transformations during cooling in α + β titanium alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- Benedetti, M.; Torresani, E.; Leoni, M.; Fontanari, V.; Bandini, M.; Pederzolli, C.; Potrich, C. The effect of post-sintering treatments on the fatigue and biological behavior of Ti-6Al-4V ELI parts made by selective laser melting. J. Mech. Behav. Biomed. 2017, 71, 295–306. [Google Scholar] [CrossRef]
- Sercombe, T.; Jones, N.; Day, R.; Kop, A. Heat treatment of Ti-6Al-7Nb components produced by selective laser melting. Rapid. Prototyp. J. 2008, 14, 300–304. [Google Scholar] [CrossRef]
- Liu, Z.; Welsch, G. Effects of oxygen and heat treatment on the mechanical properties of alpha and beta titanium alloys. Metall. Trans. A 1988, 19, 527–542. [Google Scholar] [CrossRef]
- ISO. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO, PNEN. 10993–5; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Ishimoto, T.; Hagihara, K.; Hisamoto, K.; Sun, S.-H.; Nakano, T. Crystallographic texture control of beta-type Ti–15Mo–5Zr–3Al alloy by selective laser melting for the development of novel implants with a biocompatible low Young’s modulus. Scr. Mater. 2017, 132, 34–38. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Van Humbeeck, J.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M. Phase Transformations in Metals and Alloys (Revised Reprint); CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kumar, S.S.; Pavithra, B.; Singh, V.; Ghosal, P.; Raghu, T. Tensile anisotropy associated microstructural and microtextural evolution in a metastable beta titanium alloy. Mater. Sci. Eng. A 2019, 747, 1–16. [Google Scholar] [CrossRef]
- Agarwal, N.; Bhattacharjee, A.; Ghosal, P.; Nandy, T.; Sagar, P. Heat treatment, microstructure and mechanical properties of a metastable β titanium alloy timetal® 21s. Trans. Indian Inst. Met. 2008, 61, 419–425. [Google Scholar] [CrossRef]
- Lora, C.; Rossato, G.; Jam, A.R.; Fiorese, A.; Pellizzari, M. Properties of Additive Manufactured Ti6Al4V after different vacuum heat treatments. In Proceedings of the Euro PM2019 Congress, Maastricht, The Netherlands, 13–16 October 2019. [Google Scholar]
- Sansoz, F.; Almesallmy, M.; Ghonem, H. Ductility exhaustion mechanisms in thermally exposed thin sheets of a near-β titanium alloy. Metall. Mater. Trans. A 2004, 35, 3113–3127. [Google Scholar] [CrossRef]
- Facchini, L.; Magalini, E.; Robotti, P.; Molinari, A.; Höges, S.; Wissenbach, K. Ductility of a Ti-6Al-4V alloy produced by selective laser melting of prealloyed powders. Rapid Prototyp. J. 2010, 16, 450–459. [Google Scholar] [CrossRef]
- Stráský, J.; Janeček, M.; Harcuba, P.; Preisler, D.; Landa, M. Biocompatible beta-Ti alloys with enhanced strength due to increased oxygen content. In Titanium in Medical and Dental Applications; Froes, F., Qian, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 371–392. [Google Scholar]
- Carroll, B.E.; Palmer, T.A.; Beese, A.M. Anisotropic tensile behavior of Ti–6Al–4V components fabricated with directed energy deposition additive manufacturing. Acta Mater. 2015, 87, 309–320. [Google Scholar] [CrossRef]
- Rehme, O.; Emmelmann, C. Rapid manufacturing of lattice structures with selective laser melting. In Proceedings of the Laser-Based Micropackaging, San Jose, CA, USA, 23 February 2006. [Google Scholar]
- Chen, L.; Huang, J.; Lin, C.; Pan, C.; Chen, S.; Yang, T.; Lin, D.; Lin, H.; Jang, J. Anisotropic response of Ti-6Al-4V alloy fabricated by 3D printing selective laser melting. Mater. Sci. Eng. A 2017, 682, 389–395. [Google Scholar] [CrossRef]
- Hitzler, L.; Hirsch, J.; Heine, B.; Merkel, M.; Hall, W.; Öchsner, A. On the Anisotropic Mechanical Properties of Selective Laser-Melted Stainless Steel. Materials 2017, 10, 1136. [Google Scholar] [CrossRef] [Green Version]
- Mei, Y.; Cannizzaro, C.; Park, H.; Xu, Q.; Bogatyrev, S.R.; Yi, K.; Goldman, N.; Langer, R.; Anderson, D.G. Cell-Compatible, Multicomponent Protein Arrays with Subcellular Feature Resolution. Small 2008, 4, 1600–1604. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]












| Alloy | σy0.2 (MPa) | UTS (MPa) | E (GPa) | El (%) | Structure | Reference |
|---|---|---|---|---|---|---|
| Ti–6Al–4V ELI 1,* | 1015 | 1090 | 113 | 10 | α′ | [39] |
| Ti–6Al–4V * | 990 ± 5 | 1095 ± 10 | 110 ± 5 | 8.1 ± 0.3 | α′ | [50] |
| Ti–6Al–4V + 10Mo ** | 858 ± 16 | 919 ± 10 | 73 ± 1 | 20 ± 2 | β | [34] |
| Ti–7.5Mo * | 570 | 740 | 70 | 9.2 | α + β | [35] |
| W–Ti21S 0° 2 | 852 ± 1 | 867 ± 5 | - | 16.4 ± 0.0 | β | [46] |
| W–Ti21S 45° 2 | 859 ± 11 | 884 ± 0.6 | - | 13.5 ± 0.3 | β | [46] |
| W–Ti21S 90° 2 | 797 ± 8 | 810 ± 14 | - | 16.7 ± 0.7 | β | [46] |
| β-Ti21S * | 709 ± 6 | 831 ± 3 | 52 ± 0.3 | 21 ± 1.2 | β | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellizzari, M.; Jam, A.; Tschon, M.; Fini, M.; Lora, C.; Benedetti, M. A 3D-Printed Ultra-Low Young’s Modulus β-Ti Alloy for Biomedical Applications. Materials 2020, 13, 2792. https://doi.org/10.3390/ma13122792
Pellizzari M, Jam A, Tschon M, Fini M, Lora C, Benedetti M. A 3D-Printed Ultra-Low Young’s Modulus β-Ti Alloy for Biomedical Applications. Materials. 2020; 13(12):2792. https://doi.org/10.3390/ma13122792
Chicago/Turabian StylePellizzari, Massimo, Alireza Jam, Matilde Tschon, Milena Fini, Carlo Lora, and Matteo Benedetti. 2020. "A 3D-Printed Ultra-Low Young’s Modulus β-Ti Alloy for Biomedical Applications" Materials 13, no. 12: 2792. https://doi.org/10.3390/ma13122792
APA StylePellizzari, M., Jam, A., Tschon, M., Fini, M., Lora, C., & Benedetti, M. (2020). A 3D-Printed Ultra-Low Young’s Modulus β-Ti Alloy for Biomedical Applications. Materials, 13(12), 2792. https://doi.org/10.3390/ma13122792