Insights into In Vitro Wound Closure on Two Biopolyesters—Polylactide and Polyhydroxyoctanoate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Physicochemical and Mechanical Characterization of Polymers
2.3. Cell Cultures
2.4. Cytotoxicity Assessment
2.5. Actin Cytoskeleton Staining
2.6. Quantitative Cytoskeleton Analysis
2.7. Migration Analysis
2.8. Wound Model
3. Results and Discussion
3.1. Preliminary Materials Assessment
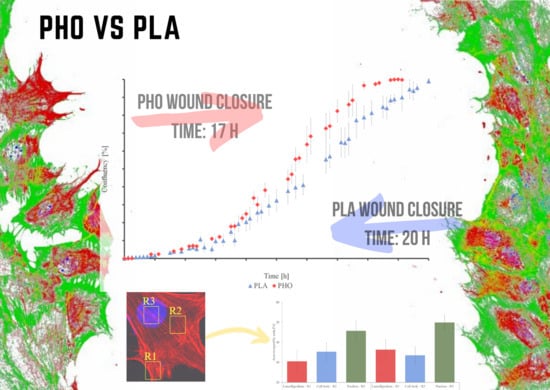
3.2. Wound Healing Model
3.3. Quantitative Cytoskeleton Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khare, S.; Prakash, O. Current Developments in Biotechnology and Bioengineering: Production, Isolation and Purification of Industrial Products; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 158, ISBN 9780444636621. [Google Scholar]
- Mittal, N.; Jansson, R.; Widhe, M.; Benselfelt, T.; Håkansson, K.M.O.; Lundell, F.; Hedhammar, M.; Söderberg, L.D. Ultrastrong and Bioactive Nanostructured Bio-Based Composites. ACS Nano 2017, 11, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Luengo, J.M.; García, B.; Sandoval, A.; Naharro, G.; Olivera, E.R. Bioplastics from microorganisms. Curr. Opin. Microbiol. 2003, 6, 251–260. [Google Scholar] [CrossRef]
- Steinbuchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Philip, S.E.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Wampfler, B.; Ramsauer, T.; Rezzonico, S.; Hischier, R.; Ko, R.; Tho, L. Isolation and Purification of Medium Chain Length Poly (3-hydroxyalkanoates) (mcl-PHA ) for Medical Applications Using Nonchlorinated Solvents. Filtration 2010, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Thermomechanical properties of polylactic acid-graphene composites: A state-of-the-art review for biomedical applications. Materials 2017, 10, 748. [Google Scholar] [CrossRef] [Green Version]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef]
- Pawar, R.P.; Tekale, S.U.; Shisodia, S.U.; Totre, J.T.; Domb, A.J. Biomedical Applications of Poly(Lactic Acid). Rec. Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Kulkarni, R.K.; Moore, E.G.; Hegyeli, A.F.; Leonard, F. Biodegradable poly(lactic acid) polymers. J. Biomed. Mater. Res. 1971, 5, 169–181. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Singh, I.; Madaan, J. Development and characterization of PLA-based green composites. J. Thermoplast. Compos. Mater. 2014, 27, 52–81. [Google Scholar] [CrossRef]
- Cichoń, E.; Haraźna, K.; Skibiński, S.; Witko, T.; Zima, A.; Ślósarczyk, A.; Zimowska, M.; Witko, M.; Leszczyński, B.; Wróbel, A.; et al. Novel bioresorbable tricalcium phosphate/polyhydroxyoctanoate (TCP/PHO) composites as scaffolds for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 98, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller II, A.L.; Waletzki, B.E.; Yaszemski, M.J.; Lu, L. Novel biodegradable poly(propylene fumarate)-co-polylactic acid) porous scaffolds fabricated by phase separation for tissue engineering applications. RSC Adv. 2015, 5, 21301–21309. [Google Scholar] [CrossRef] [Green Version]
- Amaro, L.; Correia, D.; Marques-Almeida, T.; Martins, P.; Pérez, L.; Vilas, J.; Botelho, G.; Lanceros-Mendez, S.; Ribeiro, C. Tailored Biodegradable and Electroactive Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Based Morphologies for Tissue Engineering Applications. Int. J. Mol. Sci. 2018, 19, 2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Chen, J.; Shi, M.; Zhang, H.; Ma, P.X.; Guo, B. Electroactive anti-oxidant polyurethane elastomers with shape memory property as non-adherent wound dressing to enhance wound healing. Chem. Eng. J. 2019, 375, 121999. [Google Scholar] [CrossRef]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R. Plasma surface modification of polylactic acid to promote interaction with fibroblasts. J. Mater. Sci. Mater. Med. 2013, 381, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Alsaheb, R.A.A.; Aladdin, A.; Othman, N.Z.; Malek, R.A. Recent applications of polylactic acid in pharmaceutical and medical industries. J. Chem. Pharm. Res. 2015, 7, 51–63. [Google Scholar]
- Revati, R.; Majid, M.S.A.; Normahira, M.; Unimap, P.; Tetap, K.; Putra, P. Biodegradable poly (lactic acid) scaffold for tissue engineering: A brief review. J. Polym. Sci. Technol. 2015, 1, 16–24. [Google Scholar]
- Ratner, A.; Hoffman, F.; Schoen, J.; Lemons, B. Bioresorbable and bioerodible materials. In Biomaterials Science 3rd Edition An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2012; p. 121. [Google Scholar]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Witko, T.; Solarz, D.; Feliksiak, K.; Rajfur, Z.; Guzik, M. Cellular architecture and migration behavior of fibroblast cells on polyhydroxyoctanoate (PHO): A natural polymer of bacterial origin. Biopolymers 2019. [Google Scholar] [CrossRef]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft biological materials and their impact on cell function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef]
- De Queiroz, T.S.; Prado, R.F.; Aparecida, I.; De Brito, W.; De Oliveira, L.D.; Marotta, L.; De Vasconcellos, R.; Camargo, E.A. Cytotoxicity and Genotoxicity of PLA and PCL Membranes on Osteoblasts. Acta Sci. Dent. 2019, 3, 55–59. [Google Scholar]
- Tilghman, R.W.; Cowan, C.R.; Mih, J.D.; Koryakina, Y.; Gioeli, D.; Slack-Davis, J.K.; Blackman, B.R.; Tschumperlin, D.J.; Parsons, J.T. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS ONE 2010, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kraning-Rush, C.M.; Reinhart-King, C.A. Controlling matrix stiffness and topography for the study of tumor cell migration. Cell Adhes. Migr. 2012, 6, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Rother, J.; Buchsenschutz-Gobeler, M.; Noding, H.; Steltenkamp, S.; Samwer, K.; Janshoff, A.; No, H.; Rother, J.; Bu, M.; Steltenkamp, S.; et al. Cytoskeleton remodelling of confluent epithelial cells cultured on porous substrates. J. R. Soc. Interface 2015, 12, 20141057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, C.M.; Parsons, M. Cell Migration: Developmental Methods and Protocols, 2nd ed.; Humana Press: London, UK, 2011; ISBN 9781617792069. [Google Scholar]
- Ilina, O.; Friedl, P. Mechanisms of collective cell migration at a glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, R.; Ehrlicher, A.J. The Forces Behind Cell Movement. Int. J. Biol. Sci. 2017, 3, 303–317. [Google Scholar] [CrossRef]
- Chang, S.S.; Guo, W.H.; Kim, Y.; Wang, Y.L. Guidance of cell migration by substrate dimension. Biophys. J. 2013, 104, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Puchalski, M.; Kwolek, S.; Szparaga, G.; Chrzanowski, M.; Krucinska, I. Investigation of the influence of PLA molecular structure on the crystalline forms (α’’ and α) and Mechanical Properties ofWet Spinning Fibres. Polymers 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Breen, K.C.; Ranayane, E. The effect of cell confluency state on the expression of neural cell surface glycoconjugates. Dev. Neurosci. 1994, 5, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Tamm, C.; Galitó, S.P.; Annerén, C. A comparative study of protocols for mouse embryonic stem cell culturing. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- © ibidi GmbH Manufacturer Protocol: Live/dead staining with FDA and PI. 2015. Available online: https://ibidi.com/img/cms/support/AN/AN33_Live_Dead_staining_with_FDA_and_PI.pdf (accessed on 25 November 2015).
- Forrest, L. Current concepts in soft connective tissue wound healing. Br. J. Surg. 1983, 70, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. Cell Adhesion & Migration An introduction to the wound healing assay using livecell microscopy An introduction to the wound healing assay using livecell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar]
- Lind, M. Growth factor stimulation of bone healing. Effects on osteoblasts, osteomies, and implants fixation. Acta Orthop. Scand. Suppl. 1998, 69 (Suppl. 283), 2–37. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Stewart, R.J.; Duley, J.A.; Allardyce, R.A. The migration of fibroblasts into an in vitro wound. Br. J. Exp. Pathol. 1979, 60, 582–588. [Google Scholar]
- Dorsett-martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Hopp, I.; Michelmore, A.; Smith, L.E.; Robinson, D.E.; Bachhuka, A.; Mierczynska, A.; Vasilev, K. The influence of substrate stiffness gradients on primary human dermal fibroblasts. Biomaterials 2013, 34, 5070–5077. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly Engineered PEG Hydrogels: A Novel Model System for Proteolytically Mediated Cell Migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-W.; Wu, H.-C.; Huang, Y.-C.; Sun, J.-S.; Lin, F.-H. Biomimetic Bilayered Gelatin-Chondroitin 6 Sulfate-Hyaluronic Acid Biopolymer as a Scaffold for Skin Equivalent Tissue Engineering. Artif. Organs 2006, 30, 141–149. [Google Scholar] [CrossRef]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.R.; Skodzek, K.; Hurst, S.; Mainka, A.; Steinwachs, J.; Schneider, J.; Aifantis, K.E.; Fabry, B. Biphasic response of cell invasion to matrix stiffness in three-dimensional biopolymer networks. Acta Biomater. 2015, 13, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Vu, L.T.; Jain, G.; Veres, B.D.; Rajagopalan, P. Cell Migration on Planar and Three-Dimensional Matrices: A Hydrogel-Based Perspective. Tissue Eng. Part B Rev. 2015. [Google Scholar] [CrossRef] [Green Version]
- Harley, B.A.C.; Kim, H.-D.; Zaman, M.H.; Yannas, I.V.; Lauffenburger, D.A.; Gibson, L.J. Microarchitecture of Three-Dimensional Scaffolds Influences Cell Migration Behavior via Junction Interactions. Biophys. J. 2008, 95, 4013–4024. [Google Scholar] [CrossRef] [Green Version]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef]
- Stüwe, L.; Müller, M.; Fabian, A.; Waning, J.; Mally, S.; Noël, J.; Schwab, A.; Stock, C. pH dependence of melanoma cell migration: Protons extruded by NHE1 dominate protons of the bulk solution. J. Physiol. 2007, 585, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Naveen, S.V.; Tan, I.K.P.; Goh, Y.S.; Balaji Raghavendran, H.R.; Murali, M.R.; Kamarul, T. Unmodified medium chain length polyhydroxyalkanoate (uMCL-PHA) as a thin film for tissue engineering application—Characterization and in vitro biocompatibility. Mater. Lett. 2015, 141, 55–58. [Google Scholar] [CrossRef]
- Tveitarås, M.K.; Reigstad, I.; Leiss, L.; Reed, R.K.; Stuhr, L. Single factors alone can induce mesenchymal-like morphology, but not promote full EMT in breast cancer cell lines with different hormone statuses. Exp. Cell Res. 2017, 359, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, M.; Chance, A.; Hess, E.V.; Highsmith, R.F.; FitzGerald, O. Alterations in human endothelial cell morphology, proliferation and function by a macrophage-derived factor. Ir. J. Med. Sci. 1994, 163, 359–365. [Google Scholar] [CrossRef]
- Kapitonova, M.Y.; Kuznetsov, S.L.; Froemming, G.R.A.; Muid, S.; Nor-Ashikin, M.N.K.; Otman, S.; Shahir, A.R.M.; Nawawi, H. Effects of space mission factors on the morphology and function of endothelial cells. Bull. Exp. Biol. Med. 2013, 154, 796–801. [Google Scholar] [CrossRef]
- Aw Yong, K.M.; Zeng, Y.; Vindivich, D.; Phillip, J.M.; Wu, P.-H.; Wirtz, D.; Getzenberg, R.H. Morphological Effects on Expression of Growth Differentiation Factor 15 (GDF15), a Marker of Metastasis. J. Cell. Physiol. 2014, 229, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar]





| Parameter | Cytotoxicity after 96 h of Maturation * | Single-Cell Migration Speed [um/min] | Wound Closure Time [h] | Area Occupied by Actin | ||||
|---|---|---|---|---|---|---|---|---|
| Material | Lamellipodium | Cell Body | Nucleus | Total | ||||
| PLA | 101.3% ± 1.8 | 0.63 ± 0.22 | 20.5 | 30.51 ± 4.93 | 34.73 ± 4.15 | 45.97 ± 4.42 | 34.70 ± 4.35 | |
| PHO | 99.6% ± 1.2 | 0.43 ± 0.12 | 16.4 | 35.11 ± 5.14 | 31.68 ± 4.26 | 49.66 ± 3.91 | 38.32 ± 3.12 | |
| Glass | 100% | 0.82 ± 0.21 | 16.2 | 44.75 ± 4.71 | 60.35 ± 3.89 | 51.98 ± 3.32 | 49.66 ± 3.09 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witko, T.; Solarz, D.; Feliksiak, K.; Haraźna, K.; Rajfur, Z.; Guzik, M. Insights into In Vitro Wound Closure on Two Biopolyesters—Polylactide and Polyhydroxyoctanoate. Materials 2020, 13, 2793. https://doi.org/10.3390/ma13122793
Witko T, Solarz D, Feliksiak K, Haraźna K, Rajfur Z, Guzik M. Insights into In Vitro Wound Closure on Two Biopolyesters—Polylactide and Polyhydroxyoctanoate. Materials. 2020; 13(12):2793. https://doi.org/10.3390/ma13122793
Chicago/Turabian StyleWitko, Tomasz, Daria Solarz, Karolina Feliksiak, Katarzyna Haraźna, Zenon Rajfur, and Maciej Guzik. 2020. "Insights into In Vitro Wound Closure on Two Biopolyesters—Polylactide and Polyhydroxyoctanoate" Materials 13, no. 12: 2793. https://doi.org/10.3390/ma13122793
APA StyleWitko, T., Solarz, D., Feliksiak, K., Haraźna, K., Rajfur, Z., & Guzik, M. (2020). Insights into In Vitro Wound Closure on Two Biopolyesters—Polylactide and Polyhydroxyoctanoate. Materials, 13(12), 2793. https://doi.org/10.3390/ma13122793