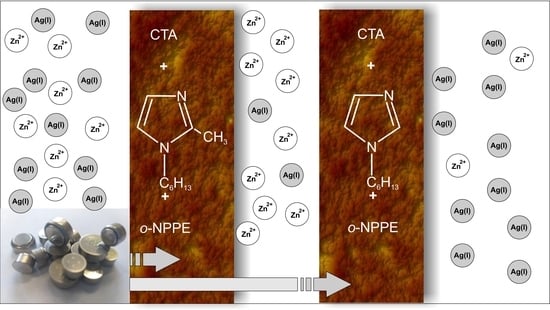
Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Polymer Inclusion Membrane
2.3. Transport Studies
3. Results
3.1. Characteristic of Membranes
3.2. Transport of Zn(II) and Ag(I) Ions across PIMs from Zn–Ag Model Solution
3.3. Recovery of Metal
3.4. Transport of Zn(II) and Ag(I) Ions across PIMs from the Ag–Zn Battery Leaching Solution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Financial Newspaper. Recycled Silver. Available online: http://archiwum.gf24.pl/srebro-z-odzysku (accessed on 3 February 2015).
- Gold Market. Recycled Silver. Available online: https://goldenmark.com/pl/mysaver/0214-srebro-odzysku/ (accessed on 8 January 2015).
- Aktas, S. Silver recovery from spent silver oxide button cells. Hydrometallurgy 2010, 104, 106–111. [Google Scholar] [CrossRef]
- Sathaiyan, N.; Nandakumar, V.; Ramachandran, P. Hydrometallurgical recovery of silver from waste silver oxide button cells. J. Power Sources 2006, 161, 1463–1468. [Google Scholar] [CrossRef]
- Gamiño-Arroyo, Z.; Tapia-Cisneros, A.; Zamacona-Saucedo, O.M.; Cano-Rodríguez, I.; Aguilera-Alvarado, A.F.; Sánchez-Cadena, L.E.; Gómez-Castro, F.I. Silver recovery from spent silver oxide button cell by liquid-liquid extraction. J. Chem. Eng. Mater. Sci. 2015, 3, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Olutoye, M.A.; Alhamdu, J.A. Electrochemical separation of metal silver from industrial wastewater. Adv. Chem. Eng. Sci. 2014, 4, 49896–49900. [Google Scholar] [CrossRef] [Green Version]
- Selim, K.A.; El Hosiny, F.I.; Abdel Khalek, M.A.; Osama, I. Kinetics and thermodynamics of some heavy metals removal from industrial effluents through electro-flotation process. Colloids Surf. Sci. 2017, 2, 47–53. [Google Scholar]
- Polat, H.; Erdogan, D. Heavy metal removal from waste waters by ion flotation. J. Hazard. Mat. 2007, 148, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Charewicz, W.A.; Holowiecka, B.A.; Walkowiak, W. Selective flotation of zinc(II) and silver(I) ions from dilute aqueous solutions. Sep. Sci. Technol. 1999, 34, 2447–2460. [Google Scholar] [CrossRef]
- Singh, R.P.; Pambid, E.R. Selective separation of silver from waste solutions on chromium(III) hexacyanoferrate(III) ion exchanger. Analyst 1990, 115, 301–304. [Google Scholar] [CrossRef]
- Akhond, M.; Absalan, G.; Sheikhian, L.; Eskandari, M.M.; Sharghi, H. Di (n-propyl) thiuram disulfide bonded on silica gel as a new sorbent for separation, preconcentration, and measurement of silver ion from aqueous samples. Sep. Purif. Technol. 2006, 52, 53–59. [Google Scholar] [CrossRef]
- Song, X.; Li, C.; Xu, R.; Wang, K. Molecular-Ion-Imprinted Chitosan Hydrogels for the Selective Adsorption of Silver(I) in Aqueous Solution. Eng. Chem. Res. 2012, 51, 11261–11265. [Google Scholar] [CrossRef]
- Hou, H.; Yu, D.; Hu, G. Preparation and properties of ion-imprinted hollow particles for the selective adsorption of silver ions. Langmuir 2015, 1, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, L.; Luo, X.; Pei, J.; Xi, Y.; Liu, C.; Liu, L. A novel non-imprinted adsorbent with superior selectivity towards high-performance capture of Ag(I). Chem. Eng. J. 2018, 348, 224–231. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V.; Singh, R.J. Review of hydrometallurgical recovery of zinc from industrial wastes. Resour. Conserv. Recy. 2001, 33, 1–22. [Google Scholar] [CrossRef]
- Winiarska, K.; Klimkiewicz, R.; Tylus, W.; Sobianowska-Turek, A.; Winiarski, J.; Szczygiel, B.; Szczygiel, I. Study of the catalytic activity and surface properties of manganese-zinc ferrite prepared from used batteries. J. Chem. 2019, 2019, 5430904. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Szczepaniak, W.; Maciejewski, P.; Gawlik-Kobylińska, M. Recovery of zinc and manganese, and other metals (Fe, Cu, Ni, Co, Cd, Cr, Na, K) from Zn-MnO2 and Zn-C waste batteries: Hydroxyl and carbonate co-precipitation from solution after reducing acidic leaching with use of oxalic acid. J. Power Sources 2016, 325, 220–228. [Google Scholar] [CrossRef]
- Chang, L.; Cao, Y.; Fan, G.; Li, C.; Peng, W. A review of the applications of ion floatation: Wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv. 2019, 35, 20226. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Ulewicz, M.; Sobianowska, K. Ion flotation and solvent sublation of zinc(II) and manganese(II) in the presence of proton-ionizable lariat ethers. Physicochem. Probl. Miner. Process. 2016, 52, 1048–1060. [Google Scholar] [CrossRef]
- Ulewicz, M.; Walkowiak, W.; Jang, Y.; Kim, J.S.; Bartsch, R.A. Ion flotation of cadmium(II) and zinc(II) in the presence of proton-ionizable lariat ethers. Anal. Chem. 2003, 75, 2276–2279. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) ions using the polymer inclusion membranes containing acetylacetone derivative as the carrier. Membranes 2020, 10, 88. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Xie, H.; Zen, X.; Li, J. Current status on leaching precious metals from waste printed circuit noards. Procedia Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Li, X.H.; Zhang, Y.H.; Hu, Q.; Wang, Z.; Fu, F. Study of spent battery material leaching process. Trans. Nonferr. Metal Soc. 2009, 19, 751–755. [Google Scholar] [CrossRef]
- Sun, P.P.; Rho, B.J.; Cho, S.Y. Recovery of silver from the nitrate leaching solution of a spent Ag/α-Al2O3 catalyst by solvent extraction and reduction. Mater. Trans. 2017, 58, 829–833. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-Y.; Xu, X.-L.; Liu, W.-Q. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef]
- Nezhadali, A.; Es’haghi Bahar, Z.S.; Banaei, A.; Shiran, J.A. Selective separation of silver(I) ion through a bulk liquid membrane containing 1,1′-(1,3-Phenylene)bis(3-allylthiourea) as carrier. J. Braz. Chem. Soc. 2016, 27, 99–108. [Google Scholar] [CrossRef]
- Mitiche, L.; Khaldoun, I.A.; Tingry, S.; Sahmoune, A. Bulk liquid membrane extraction of silver(I) with 2-mercaptobenzothiazole as a carrier. Kinetic approach. Desalin. Water Treat. 2016, 57, 18710–18717. [Google Scholar] [CrossRef]
- Othman, N.; Mat, H.; Goto, M. Separation of silver from photographic wastes by emulsion liquid membrane system. J. Membr. Sci. 2006, 282, 171–177. [Google Scholar] [CrossRef]
- Bromberg, L.; Lewin, I.; Warshawsky, A. Membrane extraction of silver by di(2-ethylhexyl) dithiophosphoric acid. J. Membr. Sci. 1992, 70, 31–39. [Google Scholar] [CrossRef]
- Amiri, A.A.; Safavi, A.; Hasaninejad, A.R.; Shrghi, H.; Shamsipur, M. Highly selective transport of silver ion through a supported liquid membrane using calix[4]pyrroles as suitable ion carriers. J. Membr. Sci. 2008, 325, 295–300. [Google Scholar] [CrossRef]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective removal of silver(I) using polymer inclusion membranes containing calixpyrroles. RSC Adv. 2019, 9, 31122–31132. [Google Scholar] [CrossRef] [Green Version]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P.; Drioli, E. Fixed sites plasticized cellulose triacetate membranes containing crown ethers for silver(I), copper(II) and gold(III) ions transport. J. Membr. Sci. 2004, 228, 149–157. [Google Scholar] [CrossRef]
- Kolodziejska, M.; Kozlowska, J.; Kozlowski, C. Separation of silver(I) and copper(II) by polymer inclusion membranes with aza[18]crown-6 derivatives as ion carriers. Desalin. Water Treat. 2017, 64, 432–436. [Google Scholar] [CrossRef]
- Shamsipur, M.; Hashemi, O.R.; Lippolis, V. A supported liquid membrane system for simultaneous separation of silver(I) and mercury(II) from dilute feed solutions. J. Membr. Sci. 2006, 282, 322–327. [Google Scholar] [CrossRef]
- Ulewicz, M.; Sadowska, K.; Biernat, J.F. Selective transport of Pb(II) across polymer inclusion membrane using imidazole azocrown ethers as carriers. Physicochem. Probl. Miner. Process. 2007, 41, 133–143. [Google Scholar]
- Kolodziejska, M.; Kozlowski, C.; Kozlowska, J.; Ulewicz, M. Selective removal of Ag(I) and Cu(II) by plasticizer membranes with N-(diethylthiophosphoryl)-aza[18]crown-6 as a carrier. Physicochem. Probl. Miner. Process. 2014, 50, 237–247. [Google Scholar] [CrossRef]
- Ulewicz, M.; Lesinska, U.; Bochenska, M. Transport of lead polymer inclusion membrane with p-tert-butylcalix[4]arene derivative. Physicochem. Probl. Miner. Process. 2010, 44, 245–256. [Google Scholar]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic membranes: Preparation and application for water treatment and desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Schulze, A.; Went, M.; Prager, M. Membrane functionalization with hyperbranched polymers. Materials 2016, 9, 706. [Google Scholar] [CrossRef] [Green Version]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Selective transport of Cu(II) across a polymer inclusion membrane with 1-alkylimidazole from nitrate solutions. Sep. Sci. Technol. 2012, 47, 1113–1118. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of the steric effect of the carrier molecule in the polymer inclusion membranes for the separation of cobalt(II), nickel(II), copper(II), and zinc(II) ions. Pol. J. Chem. Technol. 2015, 17, 51–56. [Google Scholar]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Transport of metal ions across polymer inclusion membrane with 1-alkylimidazole. Physicochem. Probl. Miner. Process. 2011, 46, 119–130. [Google Scholar]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of 1-alkylimidazoles for selective separation of zinc ions in the transport process across a polymer inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Polymer inclusion membranes (PIMs) doped with alkylimidazole and their application in the separation of non-ferrous metal ions. Polymers 2019, 11, 1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The application of polymer inclusion membranes based on CTA with 1-alkylimidazole for the separation of zinc(II) and manganese(II) ions from aqueous solutions. Polymers 2019, 11, 242. [Google Scholar] [CrossRef] [Green Version]
- Ajji, Z.; Ali, A.M. Separation of copper ions from iron ions using PVA-g-(acrylic acid/N-vinyl imidazole) membranes prepared by radiation-induced grafting. J. Hazard. Mat. 2010, 173, 71–74. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Application of polymer and supported membranes with 1-alkyl-2-methylimidazoles for separation of some transition metal ions. Desalin. Water Treat. 2017, 64, 425–431. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of supported and polymer membrane with 1-decyl-2-methylimidazole for separation of transition metal ions. Physicochem. Probl. Miner. Process. 2012, 48, 91–102. [Google Scholar]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Supported liquid (SLM) and polymer inclusion (PIM) membranes pertraction of copper(II) from aqueous nitrate solutions by 1-hexyl-2-methylimidazole. Sep. Sci. Technol. 2012, 47, 1383–1389. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. The application of membrane extraction in the separation of zinc and cadmium ions. Desalin. Water Treat. 2018, 128, 140–147. [Google Scholar] [CrossRef]
- Pernak, J.; Krysinski, J.; Skrzypczak, A. Bakterizide wirkung von iminiumverbindungen. Tenside Surfact. Det. 1987, 24, 276–286. [Google Scholar]
- Radzyminska-Lenarcik, E.; Ulewicz, R.; Ulewicz, M. Zinc recovery from model and waste solutions using polymer inclusion membrane (PIMs) with 1-octyl-4-methylimidazole. Desalin. Water Treat. 2018, 102, 211–219. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer and supported membranes with 1-decyl-4-methylimidazole for pertraction of transition metal ions. Sep. Sci. Technol. 2014, 49, 1713–1721. [Google Scholar] [CrossRef]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) transport across polymer inclusion membranes doped with imidazole azothiacrown ethers. J. Membr. Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Wolf, J.R.; Strieder, W. Tortuosities for a random fiber bed: Overlapping, parallel cylinders of several radii. J. Membr. Sci. 1990, 49, 103–115. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celikas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated transport of Cr(III) thought polymer inclusion membrane with di(2-ethylhexyl)phosphoric acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; San Miguel, E.R.; Muhammed, M.; Gyves, J. Transport characterization of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P. The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid—Base properties. J. Heterocycl. Chem. 2002, 39, 287–290. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The influence of alkyl chain length on stability Constants of Zn(II) Complexes with 1-Alkylimidazoles in Aqueous Solutions and their partition between aqueous phase and organic solvent. Solvent Extr. Ion Exch. 2004, 22, 449–471. [Google Scholar] [CrossRef]
- Barszcz, B.; Garyszewski, M.; Kulig, J. Potentiometric studies on complexes of silver(I) in solutions. Part I. Ag(I) complexes with imidazole, 1-methylimidazole and 2-methylimidazole in aqueous solutions. Pol. J. Chem. 1985, 59, 121–127. [Google Scholar]
- Lenarcik, B.; Kierzkowska, A. The influence of alkyl chain length and steric effect on extraction of zinc(II) complexes with 1-alkyl-2-methylimidazoles. Solvent Extr. Ion Exch. 2006, 24, 433–445. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Transport of metal ions across polymer inclusion membrane with 1-alkylimidazole. Physicochem. Probl. Miner. Process. 2015, 51, 447–460. [Google Scholar] [CrossRef]
- Radzymińska-Lenarcik, E.; Sulewski, M.; Urbaniak, W. Recovery of zinc from metallurgic waste sludges. Pol. J. Environ. Stud. 2015, 24, 1277–1282. [Google Scholar] [CrossRef]






| Support | Plasticizer | Carrier |
|---|---|---|
| cellulose triacetate (CTA) | o-nitrophenyl pentyl ether (o-NPPE) | 1-hexylimidazole (1) or 1-hexyl-2-methylimidazole (2) |
| Quantitative composition: 2.6 cm3 o-NPPE/1 g CTA and 1.0 mol/dm3 carriers (calculated on plasticizer) Thickness: 28–31 μm (standard deviation below 1%) | ||
| Carrier in the CTA-o-NPPE Membrane | Effective Pore Size, µm | Tortuosity | Roughness (Rq), nm |
|---|---|---|---|
| 1-hexylimidazole (1) | 0.050 ± 0.002 | 2.34 | 5.70 ± 0.05 |
| 1-hexyl-2-methylimidazole (2) | 0.053 ± 0.002 | 2.37 | 6.20 ± 0.05 |
| Carrier | Metal Ions | Initial Flux J0, μmol/m2⋅s | Selectivity Coefficients SZn(II)/Ag(I) |
|---|---|---|---|
| 1 | Zn(II) | 2.02 | Zn(II) > Ag(I) |
| Ag(I) | 1.46 | 1.38 | |
| 2 | Zn(II) | 2.09 | Zn(II) > Ag(I) |
| Ag(I) | 0.48 | 4.35 |
| Carrier | pKa [56] | Metal Ions | log β |
|---|---|---|---|
| 1 | 7.30 | Zn(II) | 5.87 [62] |
| Ag(I) | 6.33 [63] | ||
| 2 | 8.32 | Zn(II) | 5.80 [64] |
| Ag(I) | 7.14 [63] |
| Carrier | Metal Ions | RF, % |
|---|---|---|
| 1 | Zn(II) | 92 |
| Ag(I) | 90 | |
| 2 | Zn(II) | 94 |
| Ag(I) | 51 |
| Carrier | Metal Ions | J0, μmol/m2⋅s | SZn(II)/Ag(I) | RF, % |
|---|---|---|---|---|
| 1 | Zn(II) | 3.67 | 1.13 | 90 |
| Ag(I) | 3.24 | 86 | ||
| 2 | Zn(II) | 3.98 | 3.46 | 94 |
| Ag(I) | 1.15 | 47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzyminska-Lenarcik, E.; Ulewicz, M.; Pyszka, I. Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials 2020, 13, 3103. https://doi.org/10.3390/ma13143103
Radzyminska-Lenarcik E, Ulewicz M, Pyszka I. Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials. 2020; 13(14):3103. https://doi.org/10.3390/ma13143103
Chicago/Turabian StyleRadzyminska-Lenarcik, Elzbieta, Malgorzata Ulewicz, and Ilona Pyszka. 2020. "Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching" Materials 13, no. 14: 3103. https://doi.org/10.3390/ma13143103
APA StyleRadzyminska-Lenarcik, E., Ulewicz, M., & Pyszka, I. (2020). Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials, 13(14), 3103. https://doi.org/10.3390/ma13143103