Deposition of NiO Nanoparticles on Nanosized Zeolite NaY for Production of Biofuel via Hydrogen-Free Deoxygenation
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Parent Nanosized Zeolite NaY with Crystal Size of 65 nm (Y65)
2.2. Preparation of NiO-Supported Y65
2.2.1. Impregnation (IM) Method
2.2.2. Deposition–Precipitation (DP) Method with Urea
2.3. Catalyst Characterization
2.4. Catalytic Deoxygenation of Triolein
2.5. Analysis of Deoxygenated Products
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. X-ray Diffraction (XRD) Analysis
3.1.2. HRTEM Analysis
3.1.3. N2 Sorption Analysis
3.1.4. TPD-NH3 and Pyridine-FTIR Analysis
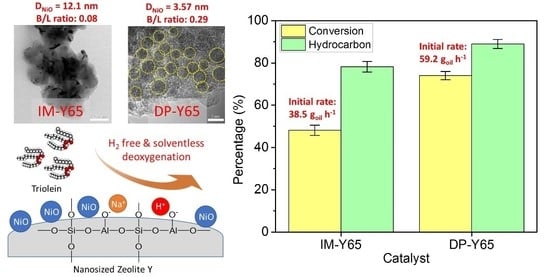
3.2. Catalytic Deoxygenation Activity
3.3. Effect of Catalyst Loading
3.4. Effect of Reaction Time
3.5. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency (IEA). Global Energy & CO2 Status Report; International Energy Agency (IEA): Paris, France, 2018. [Google Scholar]
- U.S. Energy Information Administration. Use of Energy Explained: Energy Use for Transportation 2019. Available online: https://www.eia.gov/energyexplained/use-of-energy/transportation.php (accessed on 6 November 2019).
- Hussein, A.K. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Hanaki, K.; Portugal-Pereira, J. The Effect of Biofuel Production on Greenhouse Gas Emission Reductions. In Biofuels and Sustainability: Holistic Perspectives for Policy-Making; Takeuchi, K., Shiroyama, H., Saito, O., Matsuura, M., Eds.; Springer: Tokyo, Japan, 2018; pp. 53–71. [Google Scholar]
- Choo, M.-Y.; Oi, L.E.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Ng, E.-P.; Phang, S.M.; Juan, J.C. Recent progress in catalytic conversion of microalgae oil to green hydrocarbon: A review. J. Taiwan Inst. Chem. Eng. 2017, 79 (Suppl. C), 116–124. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Liu, C.; Yan, B. Biodiesel production from palm oil using active and stable K doped hydroxyapatite catalysts. Energy Convers. Manag. 2015, 98, 463–469. [Google Scholar] [CrossRef]
- Nasreen, S.; Liu, H.; Skala, D.; Waseem, A.; Wan, L. Preparation of biodiesel from soybean oil using La/Mn oxide catalyst. Fuel Process Technol. 2015, 131, 290–296. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z. Biodiesel production from waste cooking oil by acidic ionic liquid as a catalyst. Renew. Energ. 2015, 77, 521–526. [Google Scholar] [CrossRef]
- Tan, C.H.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Lan, J.C.-W. Novel approaches of producing bioenergies from microalgae: A recent review. Biotechnol. Adv. 2015, 33 Pt 2, 1219–1227. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Chen, B.-H. High-silica zeolite beta as a heterogeneous catalyst in transesterification of triolein for biodiesel production. Catal. Today 2016, 278 Pt 2, 335–343. [Google Scholar] [CrossRef]
- Zandonai, C.H.; Yassue-Cordeiro, P.H.; Castellã-Pergher, S.B.; Scaliante, M.H.N.O.; Fernandes-Machado, N.R.C. Production of petroleum-like synthetic fuel by hydrocracking of crude soybean oil over ZSM5 zeolite—Improvement of catalyst lifetime by ion exchange. Fuel 2016, 172, 228–237. [Google Scholar] [CrossRef]
- Chandran, D. Compatibility of diesel engine materials with biodiesel fuel. Renew. Energy 2020, 147, 89–99. [Google Scholar] [CrossRef]
- Mofijur, M.; Rasul, M.; Hassan, N.M.S.; Uddin, M.N. Investigation of exhaust emissions from a stationary diesel engine fuelled with biodiesel. Energy Procedia 2019, 160, 791–797. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Assabumrungrat, S. Roles of monometallic catalysts in hydrodeoxygenation of palm oil to green diesel. Chem. Eng. J. 2015, 278, 249–258. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Juan, J.C.; Noorsaadah, A.R.; Abdulkareem-Alsultan, G.; Arumugam, M.; Taufiq-Yap, Y.H. Waste clamshell-derived CaO supported Co and W catalysts for renewable fuels production via cracking-deoxygenation of triolein. J. Anal. Appl. Pyrolysis 2016, 120, 110–120. [Google Scholar] [CrossRef]
- Ooi, X.Y.; Oi, L.E.; Choo, M.-Y.; Ong, H.C.; Lee, H.V.; Show, P.L.; Lin, Y.-C.; Juan, J.C. Efficient deoxygenation of triglycerides to hydrocarbon-biofuel over mesoporous Al2O3-TiO2 catalyst. Fuel Process Technol. 2019, 194, 106120. [Google Scholar] [CrossRef]
- Oi, L.E.; Choo, M.-Y.; Lee, H.V.; Taufiq-Yap, Y.H.; Cheng, C.K.; Juan, J.C. Catalytic deoxygenation of triolein to green fuel over mesoporous TiO2 aided by in situ hydrogen production. Int. J. Hydrog. Energy 2020, 45, 11605–11614. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Morgan, T.; Lacny, J.; Mohapatra, S.; Crocker, M. Catalytic deoxygenation of triglycerides and fatty acids to hydrocarbons over carbon-supported nickel. Fuel 2013, 103, 1010–1017. [Google Scholar] [CrossRef]
- Yang, L.; Carreon, M.A. Deoxygenation of Palmitic and Lauric Acids over Pt/ZIF-67 Membrane/Zeolite 5A Bead Catalysts. ACS Appl. Mater. Interfaces 2017, 9, 31993–32000. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C. Development of a Bimetallic Pd-Ni/HZSM-5 Catalyst for the Tandem Limonene Dehydrogenation and Fatty Acid Deoxygenation to Alkanes and Arenes for Use as Biojet Fuel. ACS Catal. 2016, 6, 4512–4525. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Zeng, J.; Liu, M.; Zhang, Y.; Liu, Z. Influence of Fe/HZSM-5 catalyst on elemental distribution and product properties during hydrothermal liquefaction of Nannochloropsis sp. Algal Res. 2018, 35, 1–9. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Liu, D.; Meng, X.; Liu, C. Hydroisomerization of n-octane over bimetallic Ni-Cu/SAPO-11 catalysts. Appl. Catal. A Gen. A 2017, 543, 274–282. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Cheng, J.; Huang, R.; Zhou, J.; Cen, K. Conversion of waste cooking oil to jet biofuel with nickel-based mesoporous zeolite Y catalyst. Bioresour. Technol. 2015, 197, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Lycourghiotis, S.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C. Nickel catalysts supported on palygorskite for transformation of waste cooking oils into green diesel. Appl. Catal. B Environ. 2019, 259, 118059. [Google Scholar] [CrossRef]
- Song, W.; Zhao, C.; Lercher, J.A. Importance of Size and Distribution of Ni Nanoparticles for the Hydrodeoxygenation of Microalgae Oil. Chem. Eur. J. 2013, 19, 9833–9842. [Google Scholar] [CrossRef]
- Marei, N.N.; Nassar, N.N.; Vitale, G. The effect of the nanosize on surface properties of NiO nanoparticles for the adsorption of Quinolin-65. Phys. Chem. Chem. Phys. 2016, 18, 6839–6849. [Google Scholar] [CrossRef]
- Cauqui, M.A.; Rodriguezizquierdo, J.M. Application of the Sol-Gel Methods to Catalyst Preparation. J. Non-Cryst. Solids 1992, 147, 724–738. [Google Scholar] [CrossRef]
- Dong, X.; Ma, X.; Xu, H.; Ge, Q. Comparative study of silica-supported copper catalysts prepared by different methods: Formation and transition of copper phyllosilicate. Catal. Sci. Technol. 2016, 6, 4151–4158. [Google Scholar] [CrossRef]
- Yan, P.; Mensah, J.; Adesina, A.; Kennedy, E.; Stockenhuber, M. Highly-dispersed Ni on BEA catalyst prepared by ion-exchange-deposition-precipitation for improved hydrodeoxygenation activity. Appl. Catal. B Environ. 2020, 267, 118690. [Google Scholar] [CrossRef]
- Burattin, P.; Che, M.; Louis, C. Characterization of the Ni(II) Phase Formed on Silica Upon Deposition−Precipitation. J. Phys. Chem. B 1997, 101, 7060–7074. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Lee, H.V.; Juan, J.C.; Noorsaadah, A.R.; Taufiq-Yap, Y.H. Catalytic deoxygenation of triglycerides to green diesel over modified CaO-based catalysts. RSC Adv. 2017, 7, 46445–46460. [Google Scholar] [CrossRef] [Green Version]
- Choo, M.-Y.; Oi, L.E.; Ling, T.C.; Ng, E.-P.; Lin, Y.-C.; Centi, G.; Juan, J.C. Deoxygenation of triolein to green diesel in the H2-free condition: Effect of transition metal oxide supported on zeolite Y. J. Anal. Appl. Pyrolysis 2020, 147, 104797. [Google Scholar] [CrossRef]
- Zulkepli, S.; Juan, J.C.; Lee, H.V.; Rahman, N.S.A.; Show, P.L.; Ng, E.P. Modified mesoporous HMS supported Ni for deoxygenation of triolein into hydrocarbon-biofuel production. Energy Convers. Manag. 2018, 165, 495–508. [Google Scholar] [CrossRef]
- Baharudin, K.B.; Taufiq-Yap, Y.H.; Hunns, J.; Isaacs, M.; Wilson, K.; Derawi, D. Mesoporous NiO/Al-SBA-15 catalysts for solvent-free deoxygenation of palm fatty acid distillate. Microporous Mesoporous Mater. 2019, 276, 13–22. [Google Scholar] [CrossRef]
- Soghrati, E.; Ong, T.K.C.; Poh, C.K.; Kawi, S.; Borgna, A. Zeolite–supported nickel phyllosilicate catalyst for CO hydrogenolysis of cyclic ethers and polyols. Appl. Catal. B Environ. 2018, 235, 130–142. [Google Scholar] [CrossRef]
- Li, W.; Zheng, J.; Luo, Y.; Da, Z. Effect of hierarchical porosity and phosphorus modification on the catalytic properties of zeolite Y. Appl. Surf. Sci. 2016, 382, 302–308. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Juan, J.C.; Oi, L.E.; Ling, T.C.; Ng, E.-P.; Rahman Noorsaadah, A.; Centi, G.; Lee, K.T. The role of nanosized zeolite Y in the H2-free catalytic deoxygenation of triolein. Catal. Sci. Technol. 2019, 9, 772–782. [Google Scholar] [CrossRef]
- Zhang, Q.; Ming, W.; Ma, J.; Zhang, J.; Wang, P.; Li, R. De novo assembly of a mesoporous beta zeolite with intracrystalline channels and its catalytic performance for biodiesel production. J. Mater. Chem. A 2014, 2, 8712–8718. [Google Scholar] [CrossRef]
- Jiao, W.Q.; Fu, W.H.; Liang, X.M.; Wang, Y.M.; He, M.-Y. Preparation of hierarchically structured Y zeolite with low Si/Al ratio and its applications in acetalization reactions. RSC Adv. 2014, 4, 58596–58607. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Liu, F.; Zhao, Y.; Xiao, T. Effect of nickel loading on the performance of nano- and micro-sized ZSM-5 catalysts for methanol to hydrocarbon conversion. Catal. Today 2018, 317, 21–28. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Liu, Q.; Wang, T.; Zhang, Q.; Ma, L. A simple method to prepare highly active and dispersed Ni/MCM-41 catalysts by co-impregnation. Catal. Commun. 2013, 42, 73–78. [Google Scholar] [CrossRef]
- Al-Ani, A.; Darton, R.J.; Sneddon, S.; Zholobenko, V. Nanostructured Zeolites: The Introduction of Intracrystalline Mesoporosity in Basic Faujasite-type Catalysts. ACS Appl. Nano Mater. 2018, 1, 310–318. [Google Scholar] [CrossRef]
- Ng, E.-P.; Tat-Lun Ng, D.; Awala, H.; Wong, K.-L.; Mintova, S. Microwave synthesis of colloidal stable AlPO-5 nanocrystals with high water adsorption capacity and unique morphology. Mater. Lett. 2014, 132, 126–129. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.-P.; Retoux, R.; Boullay, P.; Goupil, J.-M.; Valtchev, V.; Mintova, S. Template-free nanosized faujasite-type zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, Z.; Zhang, X.; Liu, J.; Zhou, J.; Cen, K. Sulfonated mesoporous Y zeolite with nickel to catalyze hydrocracking of microalgae biodiesel into jet fuel range hydrocarbons. Int. J. Hydrog. Energy 2019, 44, 1650–1658. [Google Scholar] [CrossRef]
- Berenguer, A.; Bennett, J.A.; Hunns, J.; Moreno, I.; Coronado, J.M.; Lee, A.F.; Pizarro, P.; Wilson, K.; Serrano, D.P. Catalytic hydrodeoxygenation of m-cresol over Ni2P/hierarchical ZSM-5. Catal. Today 2018, 304, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Yung, M.M.; Starace, A.K.; Mukarakate, C.; Crow, A.M.; Leshnov, M.A.; Magrini, K.A. Biomass Catalytic Pyrolysis on Ni/ZSM-5: Effects of Nickel Pretreatment and Loading. Energy Fuels 2016, 30, 5259–5268. [Google Scholar] [CrossRef]
- Romero Sarria, F.; Saussey, J.; Gallas, J.P.; Marie, O.; Daturi, M. In situ and operando IR study of adsorption sites for NH4+ active species in NOx-SCR via NH3 using a Y zeolite. In Studies in Surface Science and Catalysis; Čejka, J., Žilková, N., Nachtigall, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 821–828. [Google Scholar]
- Hermida, L.; Amani, H.; Saeidi, S.; Zuhairi Abdullah, A.; Rahman Mohamed, A. Selective acid-functionalized mesoporous silica catalyst for conversion of glycerol to monoglycerides: State of the art and future prospects. Rev. Chem. Eng. 2018, 34, 239. [Google Scholar] [CrossRef]
- Morgan, T.; Grubb, D.; Santillan-Jimenez, E.; Crocker, M. Conversion of Triglycerides to Hydrocarbons Over Supported Metal Catalysts. Top. Catal. 2010, 53, 820–829. [Google Scholar] [CrossRef]








| Samples | SBET a | Smic b | Sext c | Vmic d | Vmeso e | Vtot f | Ni Content g | Si/Al Ratio h | Na/Al Ratio h |
|---|---|---|---|---|---|---|---|---|---|
| Y65 | 661 | 599 | 62 | 0.29 | 0.20 | 0.49 | - | 1.94 | 1.04 |
| IM-Y65 | 420 | 377 | 43 | 0.14 | 0.14 | 0.28 | 9.5 | 1.92 | 0.80 |
| DP-Y65 | 503 | 379 | 124 | 0.15 | 0.29 | 0.44 | 10.2 | 1.96 | 0.43 |
| Samples | TPD-NH3 Acidity (mmol/g) | Py-FTIR Acidity (mmol/g) at 200 °C | H.F a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Medium-Strong | Total | Brönsted (B) | Lewis (L) | Total Acid (B+L) | B/L Ratio | ||
| Y65 | 1.09 | 0.82 | 2.70 | 4.62 | 0.38 | 1.39 | 1.77 | 0.27 | 0.056 |
| IM-Y65 | 1.29 | 0.92 | 1.14 | 3.35 | 0.10 | 1.18 | 1.28 | 0.08 | 0.052 |
| DP-Y65 | 0.63 | 1.94 | 2.10 | 4.67 | 0.50 | 1.72 | 2.22 | 0.29 | 0.084 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choo, M.-Y.; Oi, L.E.; Daou, T.J.; Ling, T.C.; Lin, Y.-C.; Centi, G.; Ng, E.-P.; Juan, J.C. Deposition of NiO Nanoparticles on Nanosized Zeolite NaY for Production of Biofuel via Hydrogen-Free Deoxygenation. Materials 2020, 13, 3104. https://doi.org/10.3390/ma13143104
Choo M-Y, Oi LE, Daou TJ, Ling TC, Lin Y-C, Centi G, Ng E-P, Juan JC. Deposition of NiO Nanoparticles on Nanosized Zeolite NaY for Production of Biofuel via Hydrogen-Free Deoxygenation. Materials. 2020; 13(14):3104. https://doi.org/10.3390/ma13143104
Chicago/Turabian StyleChoo, Min-Yee, Lee Eng Oi, T. Jean Daou, Tau Chuan Ling, Yu-Chuan Lin, Gabriele Centi, Eng-Poh Ng, and Joon Ching Juan. 2020. "Deposition of NiO Nanoparticles on Nanosized Zeolite NaY for Production of Biofuel via Hydrogen-Free Deoxygenation" Materials 13, no. 14: 3104. https://doi.org/10.3390/ma13143104
APA StyleChoo, M. -Y., Oi, L. E., Daou, T. J., Ling, T. C., Lin, Y. -C., Centi, G., Ng, E. -P., & Juan, J. C. (2020). Deposition of NiO Nanoparticles on Nanosized Zeolite NaY for Production of Biofuel via Hydrogen-Free Deoxygenation. Materials, 13(14), 3104. https://doi.org/10.3390/ma13143104