Physical/Chemical Properties and Resorption Behavior of a Newly Developed Ca/P/S-Based Bone Substitute Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phase Identification and Chemical Composition
2.2. Granule Morphology and Dimensional Stability in Hank’s Solution
2.3. Porosity Volume Fraction and Pore Size
2.4. pH Value
2.5. Solubility Testing in Buffered Citric Acid and TRIS-HCl Solution
2.6. Biocompatibility
2.6.1. Cytotoxicity
2.6.2. Intracutaneous Reactivity Test
2.6.3. Skin Sensitization Test
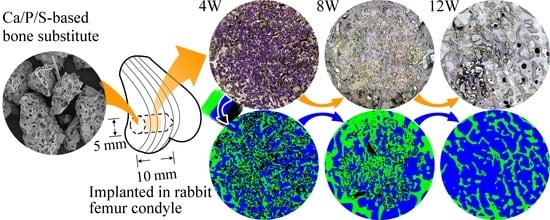
2.7. Animal Implantation Study and Resorption Rate of CBS-400 Implant
2.8. Statistical Analysis
3. Results
3.1. Phase Identification and Chemical Composition
3.2. Granule Morphology and Dimensional Stability in Hank’s Solution
3.3. Porosity Volume Fraction and Pore Size
3.4. pH Value
3.5. Solubility Tests in Buffered Citric Acid and TRIS-HCl Solutions
3.6. Biocompatibility
3.6.1. Cytotoxicity
3.6.2. Intracutaneous Reactivity Test
3.6.3. Skin Sensitization Test
3.7. Animal Implantation Study and Resorption of CBS-400 Implant
4. Discussion
5. Conclusions
- XRD patterns show that CBS-400 is comprised majorly of HA and CSD, with relatively small amounts of TTCP and DCPA. The SEM/EDS-determined Ca/P/S and Ca/P atomic ratios of the material are 54.61/39.21/6.18 and 1.39, respectively.
- Structural integrity test results show that, after immersion for 7 days, the overall morphology, shape and integrity of the Hank’s solution-immersed CBS-400 granules remain similar to that of non-immersed samples, without showing apparent collapse or disintegration under SEM.
- The average pH value of the Hank’s solution wherein CBS-400 is immersed for 1 day drops to 5.28. With immersion time, the pH value continues to increase to 6.55 after 7 days, and 7.08 after 14 days.
- In the extreme solution test, the average concentrations of Ca, P, and S in buffered citric acid solution are respectively 1208.1, 1285.4 and 570.9 ppm. In the simulation solution test, the concentrations of Ca, P, and S in TRIS-HCl are respectively 243.8, 4.6 and 443.6 ppm after immersion for 1 day; and respectively, 413.3, 14.5 and 670.2 ppm after 5 days.
- Cytotoxicity, intracutaneous reactivity and skin sensitization tests demonstrate the good biocompatibility features of CBS-400.
- The rabbit implantation results indicate that implanted CBS-400 granules are intimately bonded to the surrounding new bone at all times. The measurements of residual implant material and newly-formed cancellous bone reveal that the resorption of the implant is simultaneously replaced by the formation of new cancellous bone. During the implantation time from 3D to 12W, the average residual CBS-400 ratio decreases with time from 25.4% to 5.1%, meaning that about 85% of the implant has been resorbed after 12W. Within the same time frame, the newly formed cancellous bone ratio quickly increases to 42.4% at 4W, followed by a bone remodeling process toward normal cancellous bone, wherein the new cancellous bone ratio gradually tapers down to 30.4% after 12W.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weiser, T.G.; Haynes, A.B.; Molina, G.; Lipsitz, S.R.; Esquivel, M.M.; Uribe-Leitz, T.; Fu, R.; Azad, T.; Chao, T.E.; Berry, W.R.; et al. Size and distribution of the global volume of surgery in 2012. Bull. World Health Organ. 2016, 94, 201F–209F. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.J.; Schwartzman, A.; Zhang, J.; Liu, X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl. Health Stat. Rep. 2017, 102, 1–15. [Google Scholar]
- Deev, R.V.; Drobyshev, A.Y.; Bozo, I.Y.; Isaev, A.A. Ordinary and activated bone grafts: Applied classification and the main features. BioMed Res. Int. 2015, 2015, 365050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straumann, Annual Report 2018, Pushing Boundaries. Available online: https://www.straumann.com/content/dam/media-center/group/en/documents/annual-report/2018/2018_Straumann_Annual_report.pdf (accessed on 28 March 2019).
- Cha, H.S.; Kim, J.W.; Hwang, J.H.; Ahn, K.M. Frequency of bone graft in implant surgery. Maxillofac. Plast. Reconstr. Surg. 2016, 38, 19. [Google Scholar] [CrossRef] [Green Version]
- Egermann, M.; Schneider, E.; Evans, C.H.; Baltzer, A.W. The potential of gene therapy for fracture healing in osteoporosis. Osteoporos. Int. 2005, 16, S120–S128. [Google Scholar] [CrossRef]
- Almaiman, M.; Al-Bargi, H.H.; Manson, P. Complication of anterior iliac bone graft harvesting in 372 adult patients from may 2006 to may 2011 and a literature review. Craniomaxillofac. Trauma Reconstr. 2013, 6, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Truumees, E.; Herkowitz, H. Alternatives to autologous bone harvest in spine surgery. Univ. Pa. Orthop. J. 1999, 12, 77–88. [Google Scholar]
- Hinsenkamp, M.; Muylle, L.; Eastlund, T.; Fehily, D.; Noel, L.; Strong, D.M. Adverse reactions and events related to musculoskeletal allografts: Reviewed by the World Health Organisation Project NOTIFY. Int. Orthop. 2012, 36, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Nowzari, H.; Rich, S.K. Risk of prion disease transmission through bovine-derived bone substitutes: A systematic review. Clin. Implant. Dent. Relat. Res. 2013, 15, 645–653. [Google Scholar] [CrossRef]
- Schwartz, Z.; Weesner, T.; van Dijk, S.; Cochran, D.L.; Mellonig, J.T.; Lohmann, C.H.; Carnes, D.L.; Goldstein, M.; Dean, D.D.; Boyan, B.D. Ability of deproteinized cancellous bovine bone to induce new bone formation. J. Periodontol. 2000, 71, 1258–1269. [Google Scholar] [CrossRef]
- Murugan, R.; Rao, K.P.; Kumar, T.S.S. Heat-deproteinated xenogeneic bone from slaughterhouse waste: Physico-chemical properties. Bull. Mater. Sci. 2003, 26, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Rodriguez, A.E.; Nowzari, H. The risk of prion infection through bovine grafting materials. Clin. Implant. Dent. Relat. Res. 2016, 18, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Santin, M. 14—Bone tissue engineering. In Bone Repair Biomaterials; Planell, J.A., Best, S.M., Lacroix, D., Merolli, A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 378–422. [Google Scholar] [CrossRef]
- Blokhuis, T.J. 4—Bioresorbable bone graft substitutes. In Bone Substitute Biomaterials; Mallick, K., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 80–92. [Google Scholar] [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.; Mundt, T.; Biffar, R.; Gedrange, T.; Goetz, W. A 3-year clinical and radiographic study of implants placed simultaneously with maxillary sinus floor augmentations using a new nanocrystalline hydroxyapatite. J. Physiol. Pharmacol. 2009, 60, 91–97. [Google Scholar] [PubMed]
- Beaman, F.D.; Bancroft, L.W.; Peterson, J.J.; Kransdorf, M.J.; Menke, D.M.; DeOrio, J.K. Imaging characteristics of bone graft materials. Radiographics 2006, 26, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Huang, R.; Witt, K.L.; Southall, N.; Fostel, J.; Cho, M.-H.; Jadhav, A.; Smith, C.S.; Inglese, J.; Portier, C.J.; et al. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ. Health Perspect. 2008, 116, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Takamatsu, N. The new colorimetric assay (WST-1) for cellular growth with normal aging and Alzheimer’s disease. Nihon Ronen Igakkai Zasshi 1998, 35, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Takeyoshi, M.; Noda, S.; Yamasaki, K.; Kimber, I. Advantage of using CBA/N strain mice in a non-radioisotopic modification of the local lymph node assay. J. Appl. Toxicol. 2006, 26, 5–9. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Adam, M.; Ganz, C.; Xu, W.; Sarajian, H.-R.; Götz, W.; Gerber, T. In vivo and in vitro investigations of a nanostructured coating material—A preclinical study. Int. J. Nanomed. 2014, 9, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Bayani, M.; Torabi, S.; Shahnaz, A.; Pourali, M. Main properties of nanocrystalline hydroxyapatite as a bone graft material in treatment of periodontal defects. A review of literature. Biotechnol. Biotechnol. Equip. 2017, 31, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Levingstone, T.J.; Herbaj, S.; Dunne, N.J. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nanomaterials 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouet, C. Apatite Formation: Why it may not work as planned, and how to conclusively identify apatite compounds. BioMed. Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschhorn, J.S.; McBeath, A.A.; Dustoor, M.R. Porous titanium surgical implant materials. J. Biomed. Mater. Res. 1971, 5, 49–67. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Hulbert, S.F. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J. Biomed. Mater. Res. 1971, 5, 161–229. [Google Scholar] [CrossRef]
- Galois, L.; Mainard, D. Bone ingrowth into two porous ceramics with different pore sizes: An experimental study. Acta Orthop. Belg. 2004, 70, 598–603. [Google Scholar]
- Bohner, M.; Baumgart, F. Theoretical model to determine the effects of geometrical factors on the resorption of calcium phosphate bone substitutes. Biomaterials 2004, 25, 3569–3582. [Google Scholar] [CrossRef]
- Li, X.; van Blitterswijk, C.A.; Feng, Q.; Cui, F.; Watari, F. The effect of calcium phosphate microstructure on bone-related cells in vitro. Biomaterials 2008, 29, 3306–3316. [Google Scholar] [CrossRef]
- Coathup, M.J.; Hing, K.A.; Samizadeh, S.; Chan, O.; Fang, Y.S.; Campion, C.; Buckland, T.; Blunn, G.W. Effect of increased strut porosity of calcium phosphate bone graft substitute biomaterials on osteoinduction. J. Biomed. Mater. Res. A 2012, 100, 1550–1555. [Google Scholar] [CrossRef]
- Eidelman, N.; Chow, L.C.; Brown, W.E. Calcium phosphate phase transformations in serum. Calcif. Tissue Int. 1987, 41, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Morberg, P.; Yu, Y.; Yang, J.L.; Haggard, W.; Sheath, P.C.; Svehla, M.; Bruce, W.J. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin. Orthop. Relat. Res. 2003, 406, 228–236. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Liu, W.; Lin, K.; Pan, H.; Darvell, B.W.; Peng, S.; Wen, C.; Deng, L.; Lu, W.W.; Chang, J. Interfacial pH: A critical factor for osteoporotic bone regeneration. Langmuir 2011, 27, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C. Solubility of calcium phosphates. Monogr. Oral. Sci. 2001, 18, 94–111. [Google Scholar] [CrossRef]
- Shukla, J.; Mohandas, V.P.; Kumar, A. Effect of pH on the solubility of CaSO4·2H2O in aqueous NaCl solutions and physicochemical solution properties at 35 °C. J. Chem. Eng. Data 2008, 53, 2797–2800. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Caudill, M.A. Biochemical, Physiological, and Molecular Aspects of Human Nutrition., 3rd ed.; Elsevier: St. Louis, MO, USA, 2013; pp. 719–720. [Google Scholar]
- Finlay, J.M.; Nordin, B.E.; Fraser, R. A calcium-infusion test. II. Four-hr. skeletal retention data for recognition of osteoporosis. Lancet 1956, 270, 826–830. [Google Scholar] [CrossRef]
- Gurgan, T.; Demirol, A.; Guven, S.; Benkhalifa, M.; Girgin, B.; Li, T.C. Intravenous calcium infusion as a novel preventive therapy of ovarian hyperstimulation syndrome for patients with polycystic ovarian syndrome. Fertil. Steril. 2011, 96, 53–57. [Google Scholar] [CrossRef]
- Fischbach, F.T.; Dunning, M.B. A Manual of Laboratory and Diagnostic Tests, 9th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2015; pp. 914–983. [Google Scholar]
- van Norden, A.G.; van den Bergh, W.M.; Rinkel, G.J. Dose evaluation for long-term magnesium treatment in aneurysmal subarachnoid haemorrhage. J. Clin. Pharm. Ther. 2005, 30, 439–442. [Google Scholar] [CrossRef]
- Prieto, E.M.; Talley, A.D.; Gould, N.R.; Zienkiewicz, K.J.; Drapeau, S.J.; Kalpakci, K.N.; Guelcher, S.A. Effects of particle size and porosity on in vivo remodeling of settable allograft bone/polymer composites. J. Biomed. Mater. Res. A 2015, 103, 1641–1651. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Pao, J.L.; Chen, C.S.; Chen, Y.C.; Chang, C.C.; Hung, F.M.; Chang, C.H. Evaluation of New Biphasic Calcium Phosphate Bone Substitute: Rabbit Femur Defect Model and Preliminary Clinical Results. J. Med. Biol. Eng. 2017, 37, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M.; Galea, L.; Doebelin, N. Calcium phosphate bone graft substitutes: Failures and hopes. J. Eur. Ceram. Soc. 2012, 32, 2663–2671. [Google Scholar] [CrossRef]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in Vivo Degradation and Resorption of Calcium Phosphate Based Biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.P.; Driessen, A.A.; de Groot, K.; van den Hooff, A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J. Biomed. Mater. Res. 1983, 17, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-S.; Wu, H.-D.; Teng, N.-C.; Peng, B.-Y.; Wu, J.-Y.; Chang, W.-J.; Yang, J.-C.; Chen, C.-C.; Lee, S.-Y. In vivo evaluation of poorly crystalline hydroxyapatite-based biphasic calcium phosphate bone substitutes for treating dental bony defects. J. Dent. Sci. 2010, 5, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Xiao, J.; Wang, D.; Liu, J.; Xiong, J.; Liu, L.; Zhang, X.; Zeng, Y. Experimental study of nano-HA artificial bone with different pore sizes for repairing the radial defect. Int. Orthop. 2009, 33, 567–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Mao, K.; Liu, Z.; Wang, X.; Cui, F.; Guo, W.; Mao, K.; Yang, S. Injectable biocomposites for bone healing in rabbit femoral condyle defects. PLoS ONE 2013, 8, e75668. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Zhang, Y.L.; Tamimi, F.; Barralet, J. Effect of processing conditions of dicalcium phosphate cements on graft resorption and bone formation. Acta Biomater. 2017, 53, 526–535. [Google Scholar] [CrossRef]
- Tsai, C.H.; Lin, R.M.; Ju, C.P.; Chern Lin, J.H. Bioresorption behavior of tetracalcium phosphate-derived calcium phosphate cement implanted in femur of rabbits. Biomaterials 2008, 29, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Little, D.G.; Ramachandran, M.; Schindeler, A. The anabolic and catabolic responses in bone repair. J. Bone Joint Surg. Br. 2007, 89, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, R.; Barbieri, D.; de Groot, F.; de Bruijn, J.D.; Yuan, H. Modulating bone regeneration in rabbit condyle defects with three surface-structured tricalcium phosphate ceramics. ACS Biomater. Sci. Eng. 2018, 4, 3347–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Li, Q.; Ma, C.; Xie, D.; Li, L.; Zhao, Y.; Shan, D.; Chomos, S.K.; Dong, C.; Tierney, J.W.; et al. Development of osteopromotive poly (octamethylene citrate glycerophosphate) for enhanced bone regeneration. Acta Biomater. 2019, 93, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, O.; Goyenvalle, E.; Bouler, J.M.; Guicheux, J.; Pilet, P.; Weiss, P.; Daculsi, G. Macroporous biphasic calcium phosphate ceramics versus injectable bone substitute: A comparative study 3 and 8 weeks after implantation in rabbit bone. J. Mater. Sci.: Mater. Med. 2001, 12, 385–390. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Goyenvalle, E.; Aguado, E.; Pilet, P.; Bagot D’Arc, M.; Bilban, M.; Spaethe, R.; Daculsi, G. MBCP biphasic calcium phosphate granules and tissucol fibrin sealant in rabbit femoral defects: The effect of fibrin on bone ingrowth. J. Mater. Sci. Mater. Med. 2005, 16, 29–35. [Google Scholar] [CrossRef]
- Chakar, C.; Naaman, N.; Soffer, E.; Cohen, N.; El Osta, N.; Petite, H.; Anagnostou, F. Bone Formation with Deproteinized Bovine Bone Mineral or Biphasic Calcium Phosphate in the Presence of Autologous Platelet Lysate: Comparative Investigation in Rabbit. Int. J. Biomater. 2014, 2014, 10. [Google Scholar] [CrossRef] [Green Version]
- Sprio, S.; Sandri, M.; Panseri, S.; Iafisco, M.; Ruffini, A.; Minardi, S.; Tampieri, A. 1—Bone substitutes based on biomineralization. In Bone Substitute Biomaterials; Mallick, K., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 3–29. [Google Scholar] [CrossRef]
- Eggli, P.S.; Müller, W.; Schenk, R.K. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. A comparative histomorphometric and histologic study of bony ingrowth and implant substitution. Clin. Orthop. Relat. Res. 1988, 232, 127–138. [Google Scholar] [CrossRef]
- Ricci, J.L.; Weiner, M.J.; Iorio, D.D.; Mamidwar, S.; Alexander, H. Evaluation of timed release calcium sulfate (CS-TR) bone graft substitutes. Microsc. Microanal. 2005, 11, 1256–1257. [Google Scholar] [CrossRef] [Green Version]
- Aquino-Martínez, R.; Angelo, A.P.; Pujol, F.V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem. Cell Res. Ther. 2017, 8, 265. [Google Scholar] [CrossRef] [Green Version]
- Orsini, G.; Ricci, J.; Scarano, A.; Pecora, G.; Petrone, G.; Iezzi, G.; Piattelli, A. Bone-defect healing with calcium-sulfate particles and cement: An experimental study in rabbit. J. Biomed. Mater. Res. B. Appl. Biomater. 2004, 68, 199–208. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Bagambisa, F.B.; Joos, U.; Schilli, W. Mechanisms and structure of the bond between bone and hydroxyapatite ceramics. J. Biomed. Mater. Res. 1993, 27, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Zhu, X.; Chen, X.; Yang, X.; Zhang, K.; Fan, Y.; Zhang, X. The directional migration and differentiation of mesenchymal stem cells toward vascular endothelial cells stimulated by biphasic calcium phosphate ceramic. Regen. Biomater. 2018, 5, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, G.; Habibovic, P.; Bao, C.; Hu, J.; van Blitterswijk, C.A.; Yuan, H.; Chen, W.; Xu, H.H. The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials 2013, 34, 2167–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, T. Regulation of bone cell function by acid-base balance. Proc. Nutr. Soc. 2003, 62, 511–520. [Google Scholar] [CrossRef] [Green Version]












| Score of Polar Solvent Extraction Group | |||||||
| Animal No.1 | Animal No.2 | Animal No.3 | |||||
| Extract | Vehicle | Extract | Vehicle | Extract | Vehicle | ||
| Im. | Erythema | 0 1 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 h | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 48 h | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 72 h | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| Avg. of 3 time points (erythema + oedema) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Avg. of all extract sites (A) = 0; Avg. of all vehicle sites (B) = 0 | |||||||
| Final score of irritation of CBS-400 extract (A−B) | 0 | ||||||
| Score of Non-Polar Solvent Extraction Group | |||||||
| Animal No.1 | Animal No.2 | Animal No.3 | |||||
| Extract | Vehicle | Extract | Vehicle | Extract | Vehicle | ||
| Im. | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 h | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 48 h | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 72 h | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| Avg. of 3 time points (erythema + oedema) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Avg. of all extract sites (A) = 0; Avg. of all vehicle sites (B) = 0 | |||||||
| Final score of irritation of CBS-400 extract (A−B) | 0 | ||||||
| Score of Polar Solvent Positive Control Group | |||||||
| Animal No.1 | Animal No.2 | Animal No.3 | |||||
| Formalin | Vehicle | Formalin | Vehicle | Formalin | Vehicle | ||
| Im. | Erythema | 0 1 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 h | Erythema | 0.4 | 0 | 2.0 | 0 | 1.0 | 0 |
| Oedema | 0.6 | 0 | 3.0 | 0 | 2.0 | 0 | |
| 48 h | Erythema | 1.2 | 0 | 2.2 | 0 | 1.4 | 0 |
| Oedema | 1.6 | 0 | 3.0 | 0 | 3.0 | 0 | |
| 72 h | Erythema | 1.8 | 0 | 2.8 | 0 | 2.4 | 0 |
| Oedema | 1.2 | 0 | 2.4 | 0 | 3.0 | 0 | |
| Avg. of 3 time points (erythema + oedema) | 2.3 | 0 | 5.1 | 0 | 4.3 | 0 | |
| Avg. of all positive control sites (A) = 3.9; Avg. of all vehicle sites (B) = 0 | |||||||
| Final score of irritation of formalin (A−B) | 3.9 | ||||||
| Score of Non-Polar Solvent Positive Control Group | |||||||
| Animal No.1 | Animal No.2 | Animal No.3 | |||||
| Histamine | Vehicle | Histamine | Vehicle | Histamine | Vehicle | ||
| Im. | Erythema | 0 | 0 | 0 | 0 | 0 | 0 |
| Oedema | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 h | Erythema | 0.6 | 0 | 0.6 | 0 | 1.8 | 0 |
| Oedema | 1.4 | 0 | 0.6 | 0 | 2.4 | 0 | |
| 48 h | Erythema | 2.0 | 0 | 1.0 | 0 | 1.4 | 0 |
| Oedema | 1.4 | 0 | 1.4 | 0 | 2.6 | 0 | |
| 72 h | Erythema | 2.2 | 0 | 1.4 | 0 | 1.8 | 0 |
| Oedema | 1.4 | 0 | 1.6 | 0 | 3.0 | 0 | |
| Avg. of 3 time points (erythema + oedema) | 3.0 | 0 | 2.2 | 0 | 4.3 | 0 | |
| Avg. of all positive control sites (A) = 3.2; Avg. of all vehicle sites (B) = 0 | |||||||
| Final score of irritation of histamine (A−B) | 3.2 | ||||||
| CBS-400 Extract Group | Vehicle Control (AOO) Group | Positive Control (DNCB) Group | ||
|---|---|---|---|---|
| Body weight (g) | day 0 | 20.2 ± 0.7 | 20.5 ± 1.2 | 19.6 ± 1.3 |
| day 1 | 20.9 ± 0.5 | 20.6 ± 1.1 | 20.5 ± 1.2 | |
| day 2 | 20.3 ± 1.1 | 20.8 ± 1.2 | 20.2 ± 0.9 | |
| day 3 | 20.3 ± 0.8 | 21.0 ± 1.0 | 20.2 ± 1.0 | |
| day 4 | 20.3 ± 0.9 | 20.9 ± 1.2 | 19.5 ± 0.9 | |
| day 5 | 20.0 ± 0.8 | 20.7 ± 1.0 | 19.8 ± 0.4 | |
| Lymph nodes weight (mg) | 5.1 ± 1.3 | 4.7 ± 0.7 | 22.0 ± 2.5 * | |
| Labeling index | 0.100 ± 0.056 | 0.167 ± 0.026 | 0.431 ± 0.050 * | |
| Stimulation Index | 0.60 | 1 | 2.58 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.-C.; Lee, J.-W.; Ju, C.-P.; Chern Lin, J.-H. Physical/Chemical Properties and Resorption Behavior of a Newly Developed Ca/P/S-Based Bone Substitute Material. Materials 2020, 13, 3458. https://doi.org/10.3390/ma13163458
Yang B-C, Lee J-W, Ju C-P, Chern Lin J-H. Physical/Chemical Properties and Resorption Behavior of a Newly Developed Ca/P/S-Based Bone Substitute Material. Materials. 2020; 13(16):3458. https://doi.org/10.3390/ma13163458
Chicago/Turabian StyleYang, Bing-Chen, Jing-Wei Lee, Chien-Ping Ju, and Jiin-Huey Chern Lin. 2020. "Physical/Chemical Properties and Resorption Behavior of a Newly Developed Ca/P/S-Based Bone Substitute Material" Materials 13, no. 16: 3458. https://doi.org/10.3390/ma13163458
APA StyleYang, B. -C., Lee, J. -W., Ju, C. -P., & Chern Lin, J. -H. (2020). Physical/Chemical Properties and Resorption Behavior of a Newly Developed Ca/P/S-Based Bone Substitute Material. Materials, 13(16), 3458. https://doi.org/10.3390/ma13163458