Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Electrochemical Techniques
2.3. Microstructural Characterization
3. Results
3.1. Chemical Composition
3.2. Open Circuit Potential (OCP)
3.3. Cyclic Potentiodynamic Polarization (CPP)
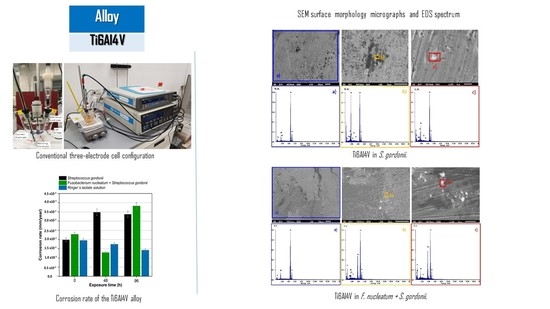
3.4. SEM Microstructural Analysis
4. Discussion
5. Conclusions
- This work presents a study on the corrosion behavior of Ti6Al4V alloy exposed with bacteria (simulating an oral environment). Their electrochemical behavior was studied by open circuit potential and cyclic potentiodynamic polarization.
- After 96 h of exposure, the Ti6Al4V alloy had the most active potentials in the three mediums because of the greater stability of the passive layer on the surface due to the formation of a passive film in the electrolyte-metal interface.
- Cyclic potentiodynamic polarization results indicated that the greater corrosion rate in Ti6Al4V alloy at 96 h of exposure is produced by the medium that contains the bacteria Streptococcus gordonii (3.38 × 10−3 mm/yr for Ti6Al4V); this is attributed to the fact that it is a primary colonizer.
- The Ti6Al4V alloy in the three solutions (Fusobacterium nucleatum + Streptococcus gordonii, Streptococcus gordonii, and Ringer’s lactate) presents a uniform corrosion behavior at different exposure times.
- SEM observations indicated that morphology does not present localized corrosion in the Ti6Al4V alloy in the three solutions Fusobacterium nucleatum + Streptococcus gordonii, Streptococcus gordonii, and Ringer’s lactate. In the Fusobacterium nucleatum + Streptococcus gordonii, Streptococcus gordonii solutions, deposits rich in silicon and carbon, derived from the microbial metabolism of casein, were identified.
- Given the enormous medical importance of these types of dental implants (the titanium alloys), and in order to obtain a better understanding of their corrosion behavior, it is recognized that the use of powerful techniques such as Electrochemical Impedance Spectroscopy, EIS, would be of great benefit.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lemons, J.; Venugopalan, R.; Lucas, L. Corrosion and Biodegradation. In Handbook of Biomaterials Evaluation; von Recum, A.F., Ed.; Taylor & Francis: New York, NY, USA, 1999; pp. 155–167. [Google Scholar]
- Yu, S. Corrosion Resistance of Titanium Alloys. In ASM Handbook Volume 13A: Corrosion: Fundamentals, Testing and Protection; ASM International: Materials Park, OH, USA, 2003. [Google Scholar]
- Al-Mayouf, M.A.; Al-Swayih, A.A.; Al-Mobarak, N.A.; Al-Jabab, S.A. Corrosion behavior of new titanium alloy for dental implant applications. Saudi Dent. J. 2002, 14, 118–125. [Google Scholar]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olson, D.L. Electrochemical Behavior of Titanium and Its Alloys as Dental Implants in Normal Saline. J. Phys. Chem. Lett. 2009, 2009, 574359. [Google Scholar] [CrossRef] [Green Version]
- Olmedo, G.D.; Tasat, R.D.; Duffó, G.; Guglielmotti, B.M.; Cabrini, L.R. The Issue of Corrosion in Dental Implants: A Review. Acta Odontol. Latinoam. 2009, 22, 3–9. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: A Multidisciplinary Endeavor. In Biomaterials Science. An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2009; pp. 1–9. [Google Scholar]
- Manresa, C.; Sanz-Miralles, E.C.; Twigg, J.; Bravo, M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst. Rev. 2018, 1, CD009376. [Google Scholar] [CrossRef] [PubMed]
- Pozhitkov, A.E.; Daubert, D.; Brochwicz, D.A.; Goodgion, D.; Vagin, M.Y.; Leroux, B.G.; Hunter, C.M.; Flemmig, T.F.; Noble, P.A.; Bryers, J.D. Interruption of Electrical Conductivity of Titanium Dental Implants Suggests a Path towards Elimination of Corrosion. PLoS ONE 2015, 10, e0140393. [Google Scholar] [CrossRef] [Green Version]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef] [Green Version]
- Herrero-Climent, M.; López-Jarana, P.; Lemos, B.F.; Gil, F.J.; Falcão, C.; Ríos-Santos, J.V.; Ríos-Carrasco, B. Relevant Design Aspects to Improve the Stability of Titanium Dental Implants. Materials 2020, 13, 1910. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, L.W.; Rockler, B.; Carlsson, G.E. Bone resorption around fixtures in edentulous patients treated with mandibular fixed tissue-integrated prostheses. J. Prosthet. Dent. 1988, 59, 59–63. [Google Scholar] [CrossRef]
- Nevins, M.; Langer, B. The successful application of osseointegrated implants to the posterior jaw: Along-term retrospective study. Int. J. Oral Maxillofac. Implant. 1993, 8, 428–432. [Google Scholar]
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased Levels of Dissolved Titanium Are Associated with Periimplantitis—A Cross-Sectional Study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef]
- Isler, S.C.; Soysal, F.; Ceyhanlı, T.; Bakırarar, B.; Unsal, B. Regenerative surgical treatment of periimplantitis using either a collagen membrane or concentrated growth factor: A 12-month randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2018, 20, 703–712. [Google Scholar] [CrossRef]
- Kommerein, N.; Doll, K.; Stumpp, N.S.; Stiesch, M. Development and characterization of an oral multispecies biofilm implant flow chamber model. PLoS ONE 2018, 13, e0196967. [Google Scholar] [CrossRef] [Green Version]
- Segura, G.; Gil, R.; Vicente, F.; Ferreiroa, A.; Faus, J.; Agustín, R. Periimplantitis y mucositis periimplantaria: Factores de riesgo; diagnóstico y tratamiento. Av. Periodoncia Implantol. Oral 2015, 27, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Hernández, M.; García-Pérez, V.I.; Almaguer-Flores, A. Potential of salivary proteins to reduce oral bacterial colonization on titanium implant surfaces. Mater. Lett. 2019, 252, 120–122. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Matos, A.O.; Ricomini-Filho, A.P.; Beline, T.; Ogawa, E.S.; Costa-Oliveira, B.E.; De Almeida, A.B.; Nociti, F.H.; Rangel, E.C.; da Cruz, N.C.; Sukotjo, C. Three-species biofilm model onto plasma-treated titanium implant surface. Colloids Surf. B Biointerfaces 2017, 152, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Rath, H.; Feng, D.; Neuweiler, I.; Stumpp, N.S.; Nackenhorst, U.; Stiesch, M. Biofilm formation by the oral pioneer colonizer Streptococcus gordonii: An experimental and numerical study. FEMS Microbiol. Ecol. 2017, 93, fix010. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Yuan, Y.; Adayi, A.; Zhang, X.; Song, X.; Gong, L.; Zhang, X.; Gao, P. Engineered chimeric peptides with antimicrobial and titanium-binding functions to inhibit biofilm formation on Ti implants. Mater. Sci. Eng. C 2018, 82, 141–154. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Han, T.J. How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int. J. Oral Maxillofac. Implant. 2007, 22, 173–202. [Google Scholar]
- Lucarini, G.; Zizzi, A.; Rubini, C. VEGF, Microvessel Density, and CD44 as Inflammation Markers in Peri-implant Healthy Mucosa, Peri-implant Mucositis, and Peri-implantitis: Impact of Age, Smoking, PPD, and Obesity. Inflammation 2019, 42, 682–689. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, N.T.; Martinez, G.A.S.; Robin, A. Electrochemical behavior of three CP titanium dental implants in artificial saliva. Mater. Res. 2009, 12, 363–366. [Google Scholar] [CrossRef]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Abdulghafoor, Y.; Abdullatif, S.; Aswad, M. Electrochemical behavior of pure titanium implanting in the teeth in acidic artificial saliva. In Proceedings of the International Conference on Education in Mathematics, Science & Technology (ICEMST), Ephesus-Kusadasi, Turkey, 18–21 May 2017. [Google Scholar] [CrossRef] [Green Version]
- Dimic, I.; Rakin, M.; Perić-Grujić, A.; Rakin, M.; Branko, B.; Putić, S.; Cvijović-Alagić, I. Effect of the pH of artificial saliva on ion release from commercially pure titanium. Acta Period. Technol. 2013, 44, 207–215. [Google Scholar] [CrossRef]
- Krupa, D.; Baszkiewicz, J.; Sobczak, J.W.; Biliński, A.; Barcz, A. Modifying the properties of titanium surface with the aim of improving its bioactivity and corrosion resistance. J. Mater. Process. Technol. 2003, 143–144, 158–163. [Google Scholar]
- Okazaki, Y.; Tateishi, T.; Ito, Y. Corrosion Resistance of Implant Alloys in Pseudo Physiological Solution and Role of Alloying Elements in Passive Films. Mater. Trans. 1997, 38, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Gil, F.J.; Ginebra, M.P.; Planell, J.A. Metales y aleaciones para la substitución de tejidos duros. Biomecánica 1999, VII, 73–78. [Google Scholar]
- Fiorillo, L.; D’amico, C.; Campagna, P.; Terranova, A.; Militi, A. Dental Materials Implant Alloys: An X-ray Fluorescence Analysis on FDS76®. Minerva Stomatol. 2020, in press. [Google Scholar]
- Blackwood, D.J.; Chua, A.W.C.; Seah, K.H.W.; Thampuran, R.; Teoh, S.H. Corrosion behaviour of porous titanium–graphite composites designed for surgical implants. Corros. Sci. 2000, 42, 481–503. [Google Scholar] [CrossRef]
- Allen, R.J.; Waclaw, B. Bacterial growth: A statistical physicist’s guide. Rep. Prog. Phys. 2018, 82, 016601. [Google Scholar] [CrossRef] [Green Version]
- ASTM G3-14 Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM G61-86 Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys; ASTM International: West Conshohocken, PA, USA, 2018.
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Van Der Woude, M.W.; Bäumler, A.J. Phase and Antigenic Variation in Bacteria. Clin. Microbiol. Rev. 2004, 17, 581–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Cabral, M.J.A.; Estupinan, F.; Baltazar-Zamora, M.A.; Almeraya-Calderon, F. Corrosion Behaviour of 304 Austenitic, 15–5PH and 17-4PH Passive Stainless Steels in acid solutions. Int. J. Electrochem. Sci. 2018, 13, 10314–10324. [Google Scholar] [CrossRef]
- Treseder, R.S. NACE Corrosion Engineers Reference Book, 2nd ed.; NACE International: Houston, TX, USA, 1991. [Google Scholar]
- Almeraya-Calderón, F.; Orozco, C.V.; Gaona-Tiburcio, C.; Borunda, T.A.; Chacón-Nava, J.; Martínez-Villafañe, A. 24—Corrosion monitoring in the pulp and paper industry. In Techniques for Corrosion Monitoring; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Cambridge, UK, 2008; pp. 584–600. [Google Scholar] [CrossRef]
- ASTM-G102-89. Standard Practice for Calculation of Corrosion Rates from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Mystkowska, J.; Niemirowicz Laskowska, K.; Łysik, D.; Tokajuk, G.; Dabrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterials surface destruction-corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Lewandowski, Z. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado-E, M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yıldız, A. Electropolymerization of poly(N-ethyl aniline) on mild steel: Synthesis, characterization and corrosion protection. Electrochim. Acta 2006, 51, 2949–2955. [Google Scholar] [CrossRef]
- Grosgogeat, B.; Reclaru, L.; Lissac, M.; Dalard, F. Measurement and evaluation of galvanic corrosion between titanium/Ti6Al4V implants and dental alloys by electrochemical techniques and auger spectrometry. Biomaterials 1999, 20, 933–941. [Google Scholar] [CrossRef]
- Revie, R.W.; Greene, N.D. Corrosion behaviour of surgical implant materials: I. effects of sterilization. Corros. Sci. 1969, 9, 755–762. [Google Scholar] [CrossRef]
- Fraker, A.C.; Ruff, A.W.; Sung, P.; Van Orden, A.C.; Speck, K.M. Surface Preparation and Corrosion Behavior of Titanium Alloys for Surgical Implants. In Titanium Alloys in Surgical Implants; ASTM International: West Conshohocken, PA, USA, 1983. [Google Scholar] [CrossRef]
- Tuna, S.H.; Pekmez, N.O.; Keyf, F.; Canlí, F. The electrochemical properties of four dental casting suprastructure alloys coupled with titanium implants. J. Appl. Oral Sci. 2009, 17, 467–475. [Google Scholar] [CrossRef]
- Mystkowska, J.; Ferreira, J.A.; Leszczyńska, K.; Chmielewska, S.; Dąbrowski, J.R.; Wieciński, P.; Kurzydłowski, K.J. Biocorrosion of 316LV steel used in oral cavity due to Desulfotomaculum nigrificans bacteria. J. Biomed. Mater. Res. Part B 2017, 105, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Ravnholt, G. Corrosion current and pH rise around titanium coupled to dental alloys. Eur. J. Oral Sci. 1988, 96, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Stams, A.J. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, V.; Nemati, M. Anaerobic reduction of sulfate in immobilized cell bioreactors, using a microbial culture originated from an oil reservoir. Biochem. Eng. J. 2006, 31, 148–159. [Google Scholar] [CrossRef]
- Souza, M.E.; Lima, L.; Lima, C.R.; Zavaglia, C.A.; Freire, C.M. Effects of pH on the electrochemical behaviour of titanium alloys for implant applications. J. Mater. Sci. Mater. Med. 2009, 20, 549–552. [Google Scholar] [CrossRef]
- Barão, V.A.R.; Mathew, M.T.; Assunção, W.G.; Yuan, J.C.-C.; Wimmer, M.A.; Sukotjo, C. Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH—An electrochemical study. Clin. Oral Implant. Res. 2012, 23, 1055–1062. [Google Scholar] [CrossRef]
- Jun, S.; Si, F.; Pugatch, R.; Scott, M. Fundamental principles in bacterial physiology—History, recent progress, and the future with focus on cell size control: A review. Rep. Prog. Phys. 2018, 81, 056601. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Nakamatsu, S.; Rueda, A.L.P.; Souza, J.J.; Rezende, S.C.D.; Souza, L.L.; Mariano, N.A. Resistance to Pitting Corrosion in Steels Based on the Fe-Cr-Ni-C System. Mater. Res. 2017, 20, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, M.M.; Rumbaugh, K.P.; Whiteley, M. Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection. PLoS Pathog. 2011, 7, e1002012. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, K.D.; Charbonneau, M.E.; O’Riordan, M.X.D. Bacterial Metabolism Shapes the Host–Pathogen Interface. Microbiol. Spectr. 2016, 4, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Berbel, L.O.; Banczek, E.d.P.; Karousis, I.K.; Kotsakis, G.A.; Costa, I. Determinants of corrosion resistance of Ti-6Al-4V alloy dental implants in an In Vitro model of peri-implant inflammation. PLoS ONE 2019, 14, e0210530. [Google Scholar] [CrossRef] [Green Version]
- Schiff, N.; Gosgpgeat, B.; Lissac, M.; Dalard, F. Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys. Biomaterials 2002, 23, 1995–2002. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.P.; Rocha, L.A. Wear and Corrosion Interactions on Titanium in Oral Environment: Literature Review. J. Bio Tribo Corros. 2015, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Shimazu, K.; Takahashi, Y.; Uchikawa, Y.; Shimazu, Y.; Yajima, A.; Takashima, E.; Aoba, T.; Konishi, K. Identification of the Streptococcus gordonii glmM gene encoding phosphoglucosamine mutase and its role in bacterial cell morphology, biofilm formation, and sensitivity to antibiotics. FEMS Immunol. Med. Microbiol. 2008, 53, 166–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Cate, J. Biofilms, a new approach to the microbiology of dental plaque. Odontology 2006, 94, 1–9. [Google Scholar] [CrossRef]
- Świder, K.; Dominiak, M.; Grzech-Leśniak, K.; Matys, J. Effect of Different Laser Wavelengths on Periodontopathogens in Peri-Implantitis: A Review of In Vivo Studies. Microorganisms 2019, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Cicciu, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]






| Exposition (h) | pH | ||
|---|---|---|---|
| Fusobacterium nucleatum + Streptococcus gordonii | Streptococcus gordonii | Ringer’s Lactate Solution | |
| 0 | 5.6 | 5.7 | 7 |
| 48 | 6.7 | 6.5 | 7 |
| 96 | 6.9 | 6.8 | 7 |
| Alloys | Elements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Si | Mn | S | C | N | Al | O | V | Ti | Fe | |
| Ti6Al4V | - | - | 0.44 | - | - | 0.04 | 0.015 | 6.1 | 0.18 | 4 | Bal | 0.183 |
| Open Circuit Potential (mV) vs. Saturated Calomel Electrode (SCE) | |||||||
|---|---|---|---|---|---|---|---|
| Fusobacterium nucleatum + Streptococcus gordonii | Streptococcus gordonii | Ringer’s Lactate Solution | |||||
| Exposure Time (h) | Material | Start | End | Start | End | Start | End |
| 0 | Ti6Al4V | −234 ±12 | −227 ±11 | −510 ±26 | −392 ±20 | −330 ±17 | −157 ±8 |
| 48 | Ti6Al4V | −264 ±13 | −287 ±14 | −472 ±24 | −461 ±23 | −294 ±15 | −17 ±0.85 |
| 96 | Ti6Al4V | −772 ±39 | −427 ±21 | −287 ±14 | −297 ±15 | −280 ±14 | −27 ±1.35 |
| Exposure Time (h) | Material | Fusobacterium gucleatum + Streptococcus gordonii | Streptococcus gordonii | Ringer’s Lactate Solution | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecorr (mV) | icorr (nA/cm2) | Corr.Rate (mm/Year) | ipass (A/cm2) | Ecorr (mV) | icorr (nA/cm2) | Corr. Rate (mm/Year) | ipass (A/cm2) | Ecorr (mV) | icorr (nA/cm2) | Corr. Rate (mm/Year) | ipass (A/cm2) | ||
| 0 | Ti6Al4V | −417 ±21 | 140 ±7 | 2.28 × 10−3 ±1 × 10−4 | 1.70 × 10−6 ±9 × 10−8 | −586 ±29 | 122 ±6 | 1.99 × 10−3 ±10 × 10−5 | 1.52 × 10−6 ±8 × 10−8 | −309 ±15 | 120 ±4 | 1.95 × 10−3 ±10 × 10−5 | 2.05 × 10−6 ±1 × 10−7 |
| 48 | Ti6Al4V | −461 ±23 | 79 ±4 | 1.29 × 10−3 ±6 × 10−5 | 1.37 × 10−6 ±7 × 10−8 | −473 ±24 | 214 ±11 | 3.49 × 10−6 ±2 × 10−4 | 4.46 × 10−6 ±2 × 10−7 | −187 ±9 | 107 ±3 | 1.74 × 10−3 ±9 × 10−5 | 1.71 × 10−6 ±9 × 10−8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Garza-Ramos, M.A.; Estupiñan-Lopez, F.H.; Gaona-Tiburcio, C.; Beltrán-Novelo, L.G.; Zambrano-Robledo, P.; Cabral-Miramontes, J.; Almeraya-Calderón, F. Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions. Materials 2020, 13, 4185. https://doi.org/10.3390/ma13184185
De la Garza-Ramos MA, Estupiñan-Lopez FH, Gaona-Tiburcio C, Beltrán-Novelo LG, Zambrano-Robledo P, Cabral-Miramontes J, Almeraya-Calderón F. Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions. Materials. 2020; 13(18):4185. https://doi.org/10.3390/ma13184185
Chicago/Turabian StyleDe la Garza-Ramos, Myriam A., Francisco H. Estupiñan-Lopez, Citlalli Gaona-Tiburcio, Lucía G. Beltrán-Novelo, Patricia Zambrano-Robledo, José Cabral-Miramontes, and Facundo Almeraya-Calderón. 2020. "Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions" Materials 13, no. 18: 4185. https://doi.org/10.3390/ma13184185
APA StyleDe la Garza-Ramos, M. A., Estupiñan-Lopez, F. H., Gaona-Tiburcio, C., Beltrán-Novelo, L. G., Zambrano-Robledo, P., Cabral-Miramontes, J., & Almeraya-Calderón, F. (2020). Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions. Materials, 13(18), 4185. https://doi.org/10.3390/ma13184185