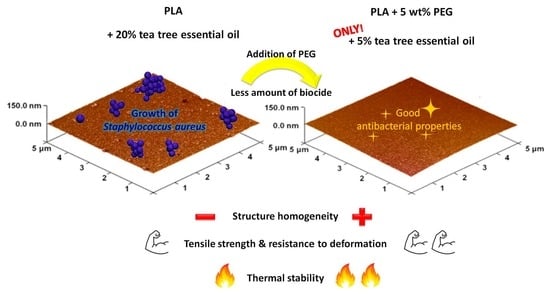
Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
2.3. Methods of Analysis
2.3.1. Scanning Electron Microscopy
2.3.2. Atomic Force Microscopy
2.3.3. Thermogravimetry
2.3.4. Differential Scanning Calorimetry
2.3.5. Uniaxial Tensile Test
2.3.6. Thickness and Transparency of Studied Materials
2.3.7. Evaluation of Antibacterial Activity
3. Results and Discussion
3.1. Assessment of Film Morphology
3.2. Examination of Surface Topography of PLA-Based Films
3.3. Determination of Thermal Properties of PLA-Based Films
3.4. Evaluation of Mechanical Properties
3.5. Thickness and Transparency of Studied PLA-Based Materials
3.6. Examination of Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, C.; Zhao, X.; Yan, H.; Meng, R.; Zhang, Y.; Li, W.; Liu, Z.; Guo, N. Effect of tea tree oil on Staphylococcus aureus growth and enterotoxin production. Food Control 2016, 62, 257–263. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. Thermo-mechanical, structural characterization and antibacterial performance of solvent casted polylactide/cinnamon oil composite films. Food Control 2016, 69, 196–204. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents 2009, 33, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Halcón, L.; Milkus, K. Staphylococcus aureus and wounds: A review of tea tree oil as a promising antimicrobial. Am. J. Infect. Control 2004, 32, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial, Rheological, and Thermal Properties of Plasticized Polylactide Films Incorporated with Essential Oils to Inhibit Staphylococcus aureus and Campylobacter jejuni. J. Food Sci. 2016, 81, E419–E429. [Google Scholar] [CrossRef]
- Mulla, M.; Ahmed, J.; Al-Attar, H.; Castro-Aguirre, E.; Arfat, Y.A.; Auras, R. Antimicrobial efficacy of clove essential oil infused into chemically modified LLDPE film for chicken meat packaging. Food Control 2017, 73, 663–671. [Google Scholar] [CrossRef]
- Sánchez-González, L.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Physical and antimicrobial properties of chitosan-tea tree essential oil composite films. J. Food Eng. 2010, 98, 443–452. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Efficacy of antimicrobial properties of polylactide/cinnamon oil film with and without high-pressure treatment against Listeria monocytogenes and Salmonella typhimurium inoculated in chicken sample. Food Packag. Shelf Life 2016, 10, 72–78. [Google Scholar] [CrossRef]
- Wabner, D.; Geier, K.; Hauck, D. For a deeper understanding of tea tree oil: Fresh is best—Why we should only use fresh oil at any concentration. Int. J. Aromather. 2006, 16, 109–115. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active Chicken Meat Packaging Based on Polylactide Films and Bimetallic Ag–Cu Nanoparticles and Essential Oil. J. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Ahmed, J.; Hiremath, N.; Jacob, H. Antimicrobial efficacies of essential oils/nanoparticles incorporated polylactide films against L. monocytogenes and S. typhimurium on contaminated cheese. Int. J. Food Prop. 2017, 20, 53–67. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Qin, Y.; Li, W.; Liu, D.; Yuan, M.; Li, L. Development of active packaging film made from poly (lactic acid) incorporated essential oil. Prog. Org. Coat. 2017, 103, 76–82. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef]
- Gallart-Mateu, D.; Largo-Arango, C.D.; Larkman, T.; Garrigues, S.; De la Guardia, M. Fast authentication of tea tree oil through spectroscopy. Talanta 2018, 189, 404–410. [Google Scholar] [CrossRef]
- Nairetti, D.; Mironescu, M.; Tita, O. Antimicrobial activity of active biodegradable starch films on pathogenic microorganisms. Ann. Rom. Soc. Cell Biol. 2014, 19, 75–80. [Google Scholar]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009, 23, 2102–2109. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Ejaz, M.; Mullah, M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. Int. J. Biol. Macromol. 2018, 107, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, S. Poly(L-lactide) microspheres with controlled crystallinity. Polym. Guildf 2001, 42, 637–643. [Google Scholar] [CrossRef]
- ISO. ISO 527-1:2020 Plastics—Determination of Tensile Properties—Part 1: General Principles; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- ISO. ISO 527-3:2019 Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- ISO. ISO 20645:2006 Textile Fabrics—Determination of Antibacterial Activity: Agar Diffusion Plate Test; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Javidi, Z.; Hosseini, S.F.; Rezaei, M. Development of flexible bactericidal films based on poly(lactic acid) and essential oil and its effectiveness to reduce microbial growth of refrigerated rainbow trout. LWT Food Sci. Technol. 2016, 72, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Samal, S.K.; Biswal, M.; Mohanty, S.; Nayak, S.K. Preparation and characterization of poly(lactic acid)/poly(ethylene oxide) blend film: Effects of poly(ethylene oxide) and poly(ethylene glycol) on the properties. Polym. Int. 2019, 68, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Holcapkova, P.; Hurajova, A.; Kucharczyk, P.; Bazant, P.; Plachy, T.; Miskolczi, N.; Sedlarik, V. Effect of polyethylene glycol plasticizer on long-term antibacterial activity and the release profile of bacteriocin nisin from polylactide blends. Polym. Adv. Technol. 2018, 29, 2253–2263. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Royo, M.; Ignacio Maté, J. Antimicrobial Activity of Whey Protein Isolate Edible Films with Essential Oils against Food Spoilers and Foodborne Pathogens. J. Food Sci. 2012, 77, M383–M390. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Jakubowska, E.; Tarach, I.; Sedlarik, V.; Pummerova, M. Antibacterial Films Based on PVA and PVA–Chitosan Modified with Poly(Hexamethylene Guanidine). Polym. Basel 2019, 11, 2093. [Google Scholar] [CrossRef] [Green Version]
- Marinello, F.; La Storia, A.; Mauriello, G.; Passeri, D. Atomic Force microscopy techniques to investigate activated food packaging materials. Trends Food Sci. Technol. 2019, 87, 84–93. [Google Scholar] [CrossRef]
- Reyes-Chaparro, P.; Gutierrez-Mendez, N.; Salas-Muñoz, E.; Ayala-Soto, J.G.; Chavez-Flores, D.; Hernández-Ochoa, L. Effect of the Addition of Essential Oils and Functional Extracts of Clove on Physicochemical Properties of Chitosan-Based Films. Int. J. Polym. Sci. 2015, 2015, 714254. [Google Scholar] [CrossRef] [Green Version]
- Jahed, E.; Khaledabad, M.A.; Almasi, H.; Hasanzadeh, R. Physicochemical properties of Carum copticum essential oil loaded chitosan films containing organic nanoreinforcements. Carbohydr. Polym. 2017, 164, 325–338. [Google Scholar] [CrossRef]
- Atarés, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Phaechamud, T.; Chitrattha, S. Pore formation mechanism of porous poly(DL-lactic acid) matrix membrane. Mater. Sci. Eng. C 2016, 61, 744–752. [Google Scholar] [CrossRef]
- Rhim, J.W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the processing methods on the performance of polylactide films: Thermocompression versus solvent casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Byun, Y.; Whiteside, S.; Thomas, R.; Dharman, M.; Hughes, J.; Kim, Y.T. The effect of solvent mixture on the properties of solvent cast polylactic acid (PLA) film. J. Appl. Polym. Sci. 2012, 124, 3577–3582. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2002, 13, 690–696. [Google Scholar] [CrossRef]
- Tábi, T.; Sajó, I.E.; Szabó, F.; Luyt, A.S.; Kovács, J.G. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Anuar, H.; Azlina, H.N.; Suzana, A.B.K.; Kaiser, M.R.; Bonnia, N.N.; Surip, S.N.; Razak, S.B.A. Effect of PEG on impact strength of PLA hybrid biocomposite. In Proceedings of the ISBEIA 2012—IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA), Bandung, Indonesia, 23–26 September 2012; pp. 473–476. [Google Scholar]
- Holcapkova, P.; Hurajova, A.; Bazant, P.; Pummerova, M.; Sedlarik, V. Thermal stability of bacteriocin nisin in polylactide-based films. Polym. Degrad. Stab. 2018, 158, 31–39. [Google Scholar] [CrossRef]





| Sample | T5% (°C) | T10% (°C) | T50% (°C) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L | 273.2 | 308.7 | 342.9 | 56.5 | 124.4 | −12.9 | 154.1 | 16.8 | 3.5 |
| LT05 | 151.5 | 296.3 | 353.9 | - | 84.0 | −11.6 | 153.3 | 23.7 | 11.6 |
| LT10 | 140.9 | 307.3 | 355.2 | - | 75.8 | −10.2 | 150.9 | 21.3 | 11.2 |
| LT20 | 140.9 | 251.3 | 356.7 | - | 73.6 | −9.3 | 151.3 | 18.8 | 10.5 |
| LP | 257.2 | 288.2 | 338.8 | 54.9 | 110.6 | −23.0 | 149.0/154.8 | 23.6 | 0.6 |
| LPT05 | 240.9 | 292.4 | 352.8 | - | 100.6 | −23.2 | 145.0/153.1 | 24.9 | 1.6 |
| LPT10 | 199.5 | 270.1 | 352.3 | - | 98.8 | −22.3 | 144.3/152.1 | 23.7 | 1.4 |
| LPT20 | 185.7 | 247.3 | 352.3 | - | 97.9 | −21.9 | 140.5/150.4 | 23.0 | 1.2 |
| Sample | ||
|---|---|---|
| L | 0.084 ± 0.002 | 0.75 ± 0.07 |
| LT05 | 0.087 ± 0.002 | 0.96 ± 0.02 |
| LT10 | 0.096 ± 0.004 | 1.12 ± 0.15 |
| LT20 | 0.112 ± 0.007 | 1.33 ± 0.21 |
| LP | 0.094 ± 0.003 | 0.81 ± 0.06 |
| LPT05 | 0.099 ± 0.004 | 1.22 ± 0.12 |
| LPT10 | 0.108 ± 0.006 | 1.49 ± 0.06 |
| LPT20 | 0.118 ± 0.005 | 1.66 ± 0.05 |
| Sample | Diameter of Inhibition Zones of Bacteria Growth (mm) | Bacteria Growth in Direct Contact with Sample | Evaluation of Antibacterial Effect 1 | |||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | S. aureus | E. coli | |
| L | 0 | 0 | medium | medium | insufficient | insufficient |
| LP | 0 | 0 | medium | medium | insufficient | insufficient |
| LT05 | 0 | 0 | weak | lack | limited | good |
| LT10 | 0 | 0 | weak | lack | limited | good |
| LT20 | 0 | 0 | weak | weak | limited | limited |
| LPT05 | 0 | 0 | lack | lack | good | good |
| LPT10 | 0 | 0 | lack | lack | good | good |
| LPT20 | 1 | 0 | lack | lack | good | good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarach, I.; Olewnik-Kruszkowska, E.; Richert, A.; Gierszewska, M.; Rudawska, A. Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films. Materials 2020, 13, 4953. https://doi.org/10.3390/ma13214953
Tarach I, Olewnik-Kruszkowska E, Richert A, Gierszewska M, Rudawska A. Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films. Materials. 2020; 13(21):4953. https://doi.org/10.3390/ma13214953
Chicago/Turabian StyleTarach, Iwona, Ewa Olewnik-Kruszkowska, Agnieszka Richert, Magdalena Gierszewska, and Anna Rudawska. 2020. "Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films" Materials 13, no. 21: 4953. https://doi.org/10.3390/ma13214953
APA StyleTarach, I., Olewnik-Kruszkowska, E., Richert, A., Gierszewska, M., & Rudawska, A. (2020). Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films. Materials, 13(21), 4953. https://doi.org/10.3390/ma13214953