Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Origin and Physicochemical Parameters of the Landfill Leachate
2.3. Apparatus and Experiment Conditions
2.4. Analytical Procedures
2.5. Response Surface Methodology
3. Results and Discussion
3.1. Physicochemical Parameters of the Landfill Leachate and K2FeO4
3.2. CCD/RSM Findings
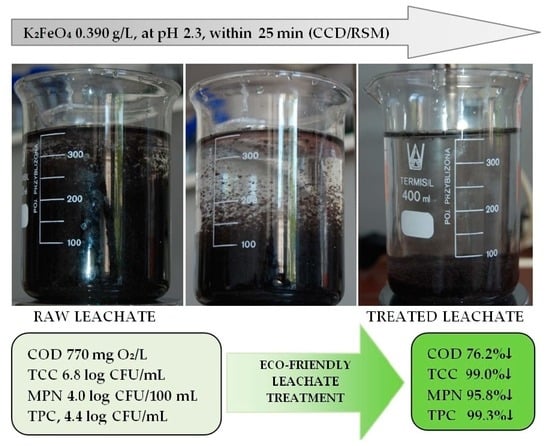
3.3. Coagulation/Flocculation Findings and K2FeO4 Biocidal Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dlugosz, J. Characteristics of the composition and quantity of leachate from municipal landfills—A review. Arch. Waste Manag. Environ. Prot. 2012, 14, 19–30. [Google Scholar]
- Xaypanya, P.; Takemura, J.; Chiemchaisri, C.; Hul, S.; Tanchuling, M.A.N. Characterization of Landfill Leachates and Sediments in Major Cities of Indochina Peninsular Countries—Heavy Metal Partitioning in Municipal Solid Waste Leachate. Environment 2018, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Banel, A.; Zygmunt, B. Volatile fatty acids in a landfill—occurrence and determination. Ecol. Chem. Eng. S. 2009, 16, 193–206. [Google Scholar]
- Renou, S.; Givaudan, J.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Urase, T.; Kikuta, T. Separate estimation of adsorption and degradation of pharmaceutical substances and estrogens in the activated sludge process. Water Res. 2005, 39, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, T.; Bautista, G.L.; Chairez, I.; Córdova, R.I.; Ríos, L.E. Decomposition of toxic pollutants in landfill leachate by ozone after coagulation treatment. J. Hazard. Mater. 2008, 152, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşlı, I.; Şafak, A.; Tünay, O. Bench-scale evaluation of treatment schemes incorporating struvite precipitation for young landfill leachate. Waste Manag. 2008, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- François, V.; Feuillade-Cathalifaud, G.; Skhiri, N.; Lagier, T.; Matejka, G. Indicating the parameters of the state of degradation of municipal solid waste. J. Hazard. Mater. 2006, 137, 1008–1015. [Google Scholar] [CrossRef]
- Öman, C.; Junestedt, C. Chemical characterization of landfill leachates—400 parameters and compounds. Waste Manag. 2008, 28, 1876–1891. [Google Scholar] [CrossRef]
- Budi, S.; Suliasih, B.A.; Othman, M.S.; Heng, L.Y.; Surif, S. Toxicity identification evaluation of landfill leachate using fish, prawn and seed plant. Waste Manag. 2016, 55, 231–237. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Ozonek, J.; Pasieczna-Patkowska, S. Application of hydrodynamic cavitation to improve the biodegradability of mature landfill leachate. Ultrason. Sonochem. 2015, 26, 378–387. [Google Scholar] [CrossRef]
- Civan, F.; Özaltun, D.H.; Kıpçak, E.; Akgün, M. The treatment of landfill leachate over Ni/Al2O3 by supercritical water oxidation. J. Supercrit. Fluids 2015, 100, 7–14. [Google Scholar] [CrossRef]
- Theepharaksapan, S.; Chiemchaisri, C.; Chiemchaisri, W.; Yamamoto, K. Removal of pollutants and reduction of bio-toxicity in a full scale chemical coagulation and reverse osmosis leachate treatment system. Bioresour. Technol. 2011, 102, 5381–5388. [Google Scholar] [CrossRef]
- Mnif, S.; Zayen, A.; Karray, F.; Bru-Adan, V.; Loukil, S.; Godon, J.J.; Chamkha, M.; Sayadi, S. Microbial population changes in anaerobic membrane bioreactor treating landfill leachate monitored by single-strand conformation polymorphism analysis of 16S rDNA gene fragments. Int. Biodeterior. Biodegrad. 2012, 73, 50–59. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Guo, J.; Wang, Z.; Feng, Q. Landfill leachate treatment using electrocoagulation. Procedia Environ. Sci. 2011, 10, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Qu, J.; Liu, H.; Wang, C.; Xiao, S.; Liu, R.; Liu, P.; Lan, H.; Hu, C. Photoelectrochemical treatment of landfill leachate in a continuous flow reactor. Bioresour. Technol. 2010, 101, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Englehardt, J.D. Electrochemical oxidation for landfill leachate treatment. Waste Manag. 2007, 27, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Wang, K.; Zhao, Q.; Wei, L.-L.; Zhang, J.; Yang, J.-C. Characterization of dissolved organic matter during landfill leachate treatment by sequencing batch reactor, aeration corrosive cell-Fenton, and granular activated carbon in series. J. Hazard. Mater. 2010, 179, 1096–1105. [Google Scholar] [CrossRef]
- Ben Yahmed, A.; Saidi, N.; Trabelsi, I.; Murano, F.; Dhaifallah, T.; Bousselmi, L.; Ghrabi, A. Microbial characterization during aerobic biological treatment of landfill leachate (Tunisia). Desalination 2009, 246, 378–388. [Google Scholar] [CrossRef]
- Mariam, T.; Guo, W. Landfill leachate treatment using hybrid coagulation-nanofiltration processes. Desalination 2010, 250, 677–681. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Ozonation as polishing treatment of mature landfill leachate. J. Hazard. Mater. 2010, 182, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Ziyang, L.; Tajuddin, R.M.; Farraji, H.; Alifar, N. Co-treatment of landfill leachate and municipal wastewater using the ZELIAC/zeolite constructed wetland system. J. Environ. Manag. 2016, 166, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Niu, C.-G.; Chen, G.; Dong, H.; Liu, Y.; Wan, J.; Chen, A.; Guo, Z.; Yan, M.; Wu, H.; et al. Treatment of landfill leachate using immobilized Phanerochaete chrysosporium loaded with nitrogen-doped TiO 2 nanoparticles. J. Hazard. Mater. 2016, 301, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Ziyang, L.; Zhao, Y.; Tao, Y.; Yu, S.; Huili, C.; Nanwen, Z.; Renhua, H. Natural attenuation and characterization of contaminants composition in landfill leachate under different disposing ages. Sci. Total. Environ. 2009, 407, 3385–3391. [Google Scholar] [CrossRef]
- Oz, N.A.; Yarimtepe, C.C. Ultrasound assisted biogas production from landfill leachate. Waste Manag. 2014, 34, 1165–1170. [Google Scholar] [CrossRef]
- Wu, L.; Peng, C.; Zhang, S.; Peng, Y. Nitrogen removal via nitrite from municipal landfill leachate. J. Environ. Sci. 2009, 21, 1480–1485. [Google Scholar] [CrossRef]
- Kargi, F.; Catalkaya, E.C. Electrohydrolysis of landfill leachate organics for hydrogen gas production and COD removal. Int. J. Hydrog. Energy 2011, 36, 8252–8260. [Google Scholar] [CrossRef]
- Tsarpali, V.; Dailianis, S. Investigation of landfill leachate toxic potency: An integrated approach with the use of stress indices in tissues of mussels. Aquat. Toxicol. 2012, 58–65. [Google Scholar] [CrossRef]
- Isaka, K.; Yoshie, S.; Sumino, T.; Inamori, Y.; Tsuneda, S. Nitrification of landfill leachate using immobilized nitrifying bacteria at low temperatures. Biochem. Eng. J. 2007, 37, 49–55. [Google Scholar] [CrossRef]
- Tatsi, A.; Zouboulis, A.; Matis, K.; Samaras, P. Coagulation–flocculation pretreatment of sanitary landfill leachates. Chemosphere 2003, 53, 737–744. [Google Scholar] [CrossRef]
- Jones, D.; Williamson, K.; Owen, A. Phytoremediation of landfill leachate. Waste Manag. 2006, 26, 825–837. [Google Scholar] [CrossRef]
- Lema, J.M.; Mendez, R.; Blazquez, R. Characteristics of landfill leachates and alternatives for their treatment: A review. Water Air Soil Pollut. 1988, 40, 223–250. [Google Scholar]
- Tatsi, A.; Zouboulis, A.I. A field investigation of the quantity and quality of leachate from a municipal solid waste landfill in a Mediterranean climate (Thessaloniki, Greece). Adv. Environ. Res. 2002, 6, 207–219. [Google Scholar] [CrossRef]
- Salem, Z.; Hamouri, K.; Djemaa, R.; Allia, K. Evaluation of landfill leachate pollution and treatment. Desalination 2008, 220, 108–114. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Płonka, I.; Barbusiński, K.; Amalio-Kosel, M. Comparison of Landfill Leachate Treatment Efficiency Using the Advanced Oxidation Processes. Arch. Environ. Prot. 2013, 39, 107–115. [Google Scholar] [CrossRef]
- Barbusiński, K.; Pieczykolan, B. COD removal from landfill leachate using Fenton oxidation and coagulation. Archit. Civ. Eng. Environ. 2010, 4, 93–100. [Google Scholar]
- Pieczykolan, K.B.B. COD removal from landfill leachate using H2O2, UV radiation and combination these processes. Environ. Prot. Eng. 2012, 38, 5–12. [Google Scholar] [CrossRef]
- Pieczykolan, B.; Barbusiński, K.; Płonka, I. Effect of landfill leachate on the biological treatment of wastewater. Przem. Chem. 2011, 90, 1555–1559. [Google Scholar]
- Barbusiński, K.; Pieczykolan, B.; Kościelniak, H.; Amalio, M. Effect of landfill leachate on the efficiency of municipal sewage treatment and on the properties of activated sludge. Ochrona Srodowiska 2010, 32, 33–38. [Google Scholar]
- Kulikowska, D. Characterization of organics and methods treatment of leachate from stabilized municipal landfills. Ecol. Chem. Eng. S. 2009, 16, 389–402. [Google Scholar]
- Paxéus, N. Organic compounds in municipal landfill leachates. Water Sci. Technol. 2000, 42, 323–333. [Google Scholar] [CrossRef]
- Sharma, V.K. Potassium ferrate (VI): An environmentally friendly oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
- Kliś, S.; Barbusiński, K.; Thomas, M.; Mochnacka, A. Application of potassium ferrate (VI) in the treatment of selected water and wastewater pollutants—Short review. Arch. Civ. Eng. Environ. 2020, 13, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Kliś, S.; Barbusiński, K.; Thomas, M.; Mochnacka, A. Application of potassium ferrate (VI) for oxidation of selected pollutants in aquatic environment—Short review. Arch. Civ. Eng. Environ. 2019, 12, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Zdebik, D. Treatment of Real Textile Wastewater by Using Potassium Ferrate (VI) and Fe (III)/H2O2. Application of Aliivibrio Fischeri and Brachionus plicatilis Tests for Toxicity Assessment. Fibres Text. East. Eur. 2019, 27, 78–84. [Google Scholar] [CrossRef]
- Thomas, M.; Barbusinski, K.; Kliś, S.; Szpyrka, E.; Chyc, M. Synthetic Textile Wastewater Treatment using Potassium Ferrate (VI)—Application of Taguchi Method for Optimisation of Experiment. Fibres Text. East. Eur. 2018, 26, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Schreyer, J.M.; Thompson, G.W.; Ockerman, L.T. Oxidation of Chromium (III) with Potassium Ferrate (VI). Anal. Chem. 1950, 22, 1426–1427. [Google Scholar] [CrossRef]
- Wei, Y.-L.; Wang, Y.-S.; Liu, C.-H. Preparation of Potassium Ferrate from Spent Steel Pickling Liquid. Metals 2015, 5, 1770. [Google Scholar] [CrossRef] [Green Version]
- ISO. 10523:2008 Water Quality. Determination of pH. Available online: https://www.iso.org/standard/51994.html (accessed on 5 October 2020).
- ISO. 15705:2002 Water Quality. Determination of the Chemical Oxygen Demand Index (ST-COD). Small-Scale Sealed-Tube Method. Available online: https://www.iso.org/standard/28778.html (accessed on 5 October 2020).
- Test 985094. TOC 60. Macherey-Nagel GmbH & Co. KG 5, Düren, German. Available online: http://ftp.mn-net.com/english/Instruction_leaflets/NANOCOLOR/985094en.PDF (accessed on 10 August 2020).
- Test 985088. Total Nitrogen TNb 220. Macherey-Nagel GmbH & Co. KG 5, Düren, Germany. Available online: https://www.mn-net.com/media/pdf/1a/bf/a1/Instruction-985088-Tube-test-NANOCOLOR-total-Nitrogen-TNb-220.PDF (accessed on 10 August 2020).
- Test 985080. Ortho- and Total Phosphat 15. Macherey-Nagel GmbH & Co. KG 5, Düren, Germany. Available online: https://www.mn-net.com/media/pdf/a8/a4/4c/Instruction-985080-Tube-test-NANOCOLOR-ortho-and-total-Phosphate-15.PDF (accessed on 10 August 2020).
- ISO 6887-1:2017. Microbiology of the Food Chain-Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination-Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. Available online: https://www.iso.org/standard/63335.html (accessed on 5 October 2020).
- ISO 4832:2006. Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Coliforms-Colony-Count Technique. Available online: https://www.iso.org/standard/38282.html (accessed on 5 October 2020).
- PN-75/C-04615/17:1975 Microbiological Tests. Determination of Proteolytic Bacteria by Frazier’s Method; The Polish Committee for Standardization: Warsaw, Poland, 1975.
- PN-C-04615-25:2008 Water and Wastewater. Microbiological Tests. Part 25. Determination of Fecal Enterococci by Tube Test; The Polish Committee for Standardization: Warsaw, Poland, 2008.
- Grisey, E.; Belle, E.; Dat, J.; Mudry, J.; Aleya, L. Survival of pathogenic and indicator organisms in groundwater and landfill leachate through coupling bacterial enumeration with tracer tests. Desalination 2010, 261, 162–168. [Google Scholar] [CrossRef]
- Kozik, V.; Barbusinski, K.; Thomas, M.; Sroda, A.; Jampilek, J.; Sochanik, A.; Smolinski, A.; Bak, A. Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate. Materials 2019, 12, 3784. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Barbusinski, K.; Kalemba, K.; Piskorz, P.J.; Kozik, V.; Bak, A. Optimization of the Fenton Oxidation of Synthetic Textile Wastewater using Response Surface Methodology. Fibres Text. East. Eur. 2017, 25, 108–113. [Google Scholar] [CrossRef]
- Aslani, H.; Nabizadeh, R.; Nasseri, S.; Mesdaghinia, A.; Alimohammadi, M.; Mahvi, A.H.; Rastkari, N.; Nazmara, S. Application of response surface methodology for modeling and optimization of trichloroacetic acid and turbidity removal using potassium ferrate (VI). Desalin. Water Treat. 2016, 57, 25317–25328. [Google Scholar] [CrossRef]
- Lan, S.; Liu, X.; Chen, R.; Wan, Y.; Wu, X.; Zhang, H. Study on pretreatment of landfill leachate by potassium ferrate. Desalin. Water Treat. 2013, 52, 2757–2764. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Z.; Qu, Y.; Lin, Y.; Huang, D.; Zheng, Z. Pretreatment of landfill leachate by potassium ferrate (VI). Chin. J. Environ. Eng. 2014, 8, 2451–2455. [Google Scholar]
- Jiang, J.-Q.; Wang, S.; Panagoulopoulos, A. The exploration of potassium ferrate (VI) as a disinfectant/coagulant in water and wastewater treatment. Chemosphere 2006, 63, 212–219. [Google Scholar] [CrossRef]
- Sharma, V.K. Desinfection performance of Fe (VI) in water and wastewater: A review. Water Sci. Technol. 2007, 55, 225–232. [Google Scholar] [CrossRef]
- Sánchez-Carretero, A.; Sáez, C.; Cañizares, P.; Cotillas, S.; Rodrigo, M.A. Improvements in the Electrochemical Production of Ferrates with Conductive Diamond Anodes Using Goethite as Raw Material and Ultrasound. Ind. Eng. Chem. Res. 2011, 50, 7073–7076. [Google Scholar] [CrossRef]
- Ghernaout, D.; Naceur, M.W. Ferrate (VI): In situ generation and water treatment—A review. Desalin. Water Treat. 2011, 30, 319–332. [Google Scholar] [CrossRef]




| Run | Experimental Conditions | Experimental Results * | ||
|---|---|---|---|---|
| pH | K2FeO4 (g/L) | Time (min) | COD (g O2/L) | |
| 1 | 2.00 | 0.200 | 10.00 | 0.385 ± 0.058 |
| 2 | 2.00 | 0.200 | 20.00 | 0.355 ± 0.053 |
| 3 | 2.00 | 0.400 | 10.00 | 0.295 ± 0.044 |
| 4 | 2.00 | 0.400 | 20.00 | 0.260 ± 0.039 |
| 5 | 5.00 | 0.200 | 10.00 | 0.465 ± 0.070 |
| 6 | 5.00 | 0.200 | 20.00 | 0.450 ± 0.068 |
| 7 | 5.00 | 0.400 | 10.00 | 0.300 ± 0.045 |
| 8 | 5.00 | 0.400 | 20.00 | 0.295 ± 0.044 |
| 9 | 0.98 | 0.300 | 15.00 | 0.245 ± 0.037 |
| 10 | 6.02 | 0.300 | 15.00 | 0.495 ± 0.074 |
| 11 | 3.50 | 0.132 | 15.00 | 0.695 ± 0.104 |
| 12 | 3.50 | 0.468 | 15.00 | 0.205 ± 0.031 |
| 13 | 3.50 | 0.300 | 6.59 | 0.345 ± 0.052 |
| 14 | 3.50 | 0.300 | 23.41 | 0.240 ± 0.036 |
| 15 (C) | 3.50 | 0.300 | 15.00 | 0.265 ± 0.040 |
| 16 (C) | 3.50 | 0.300 | 15.00 | 0.275 ± 0.041 |
| Parameter | Unit | Result * |
|---|---|---|
| pH | – | 8.9 ± 0.1 |
| Chemical Oxygen Demand, COD | mg O2/L | 770 ± 116 |
| Total Organic Carbon, TOC | mg/L | 230 ± 35 |
| Total Nitrogen, TN | mg/L | 120 ± 18 |
| Total Phosphorus, TP | mg/L | 12 ± 2 |
| Total Coli Count, TCC | CFU/mg/L | 6.2 × 106 (6.8 log) |
| Most Probable Number of fecal enterococci, MPNfe | MPN/100 mL | 1.1 × 104 (4.0 log) |
| Total Proteolytic Count, TPC | CFU/mL | 2.6 × 104 (4.4 log) |
| Parameter | Evaluation of the Effects, COD, g O2/L, R2 = 0.8477, R2adj = 0.7462, 3 Parameter, 1 Block, 16 Experiments, MS = 0.0040 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Standard Error | p-Value * | −95% Confidence Interval | +95% Confidence Interval | Factor | Standard Error of Factor | Lower Confidence Interval | Upper Confidence Interval | |
| Constant Value | 0.2725 | 0.0448 | 0.0002 | 0.1713 | 0.3738 | 0.2725 | 0.0448 | 0.1713 | 0.3738 |
| pH (L) | 0.0931 | 0.0344 | 0.0240 | 0.0153 | 0.1708 | 0.0465 | 0.0172 | 0.0077 | 0.1708 |
| pH (Q) | 0.0584 | 0.0417 | 0.1947 | −0.0359 | 0.1528 | 0.0292 | 0.0209 | −0.0180 | 0.1528 |
| K2FeO4 (L) | 0.1946 | 0.0344 | 0.0003 | −0.2724 | −0.1169 | −0.0973 | 0.0172 | −0.1362 | −0.1169 |
| K2FeO4 (Q) | 0.1150 | 0.0417 | 0.0222 | 0.0207 | 0.2094 | 0.0575 | 0.0209 | 0.0103 | 0.2094 |
| Time (L) | 0.0383 | 0.0344 | 0.2937 | −0.1160 | 0.0394 | −0.0192 | 0.0172 | −0.0580 | 0.0394 |
| Time (Q) | 0.0036 | 0.0417 | 0.9323 | −0.0907 | 0.0980 | 0.0018 | 0.0209 | −0.0454 | 0.0980 |
| Parameter | Assessment of Effects, COD, g O2/L, R2 = 0.8477, R2adj = 0.7462, 3 Parameter, 1 Block, 16 Experiments, MS = 0.0040 | |||
|---|---|---|---|---|
| SS | MS | F | p * | |
| pH (L) | 0.029567 | 0.029567 | 7.33757 | 0.024045 |
| pH (Q) | 0.007911 | 0.007911 | 1.96337 | 0.194681 |
| K2FeO4 (L) | 0.129345 | 0.129345 | 32.09914 | 0.000307 |
| K2FeO4 (Q) | 0.030637 | 0.030637 | 7.60318 | 0.022207 |
| Time (L) | 0.005011 | 0.005011 | 1.24345 | 0.293696 |
| Time (Q) | 0.000031 | 0.000031 | 0.00764 | 0.932269 |
| Error | 0.036266 | 0.004030 | – | – |
| Predictor | Regression Coefficient | Standard Error | t-Value, df = 9 | p-Value | −95% Confidence Interval | +95% Confidence Interval |
|---|---|---|---|---|---|---|
| Intercept | 1.206468 | 0.354637 | 3.401978 | 0.007849 | 0.404223 | 2.008712 |
| pH (L) | −0.059897 | 0.065887 | −0.909076 | 0.387006 | −0.208944 | 0.089151 |
| pH (Q) | 0.012988 | 0.009269 | 1.401202 | 0.194681 | −0.007980 | 0.033957 |
| K2FeO4 (L) | −4.423640 | 1.263080 | −3.502263 | 0.006700 | −7.280926 | −1.566353 |
| K2FeO4 (Q) | 5.750741 | 2.085576 | 2.757387 | 0.022207 | 1.032839 | 10.468642 |
| Time (L) | −0.006018 | 0.025262 | −0.238234 | 0.817035 | −0.063164 | 0.051128 |
| Time (Q) | 0.000073 | 0.000834 | 0.087398 | 0.932269 | −0.001814 | 0.001960 |
| Parameter * | Unit | After K2FeO4 Application in Optimal Conditions ** | After FeSO4 × 7H2O Application *** | After FeCl × 6H2O Application **** |
|---|---|---|---|---|
| Removal, % (in Brackets) ***** | ||||
| pH | – | 9.0 ± 0.1 | 9.0 ± 0.1 | 9.0 ± 0.1 |
| Chemical Oxygen Demand | mg O2/L | 180 ± 27 (↓76.2) | 475 ± 71 (↓38.1) | 450 ± 68 (↓41.6) |
| Total Organic Carbon | mg/L | 40 ± 6 (↓82.6) | 145 ± 22 (↓37.0) | 125 ± 19 (↓45.7) |
| Total Nitrogen, TN | mg/L | 38 ± 6 (↓68.3) | 95 ± 14 (↓20.8) | 85 ± 13 (↓29.2) |
| Total Phosphorus, TP | mg/L | 1.0 ± 0.2 (↓91.6) | 0.50 ± 0.08 (↓95.8) | 0.5 ± 0.08 (↓95.8) |
| Total Coli Count, TCC | CFU/mL | 5.9 × 102; 2.8 log (↓99.9) | 3.5 × 105; 5.5 log (↓94.4) | 4.9 × 105; 5.7 log (↓92.1) |
| Most Probable Number of fecal enterococci, MPN | MPN/100 mL | 4.6 × 102; 2.7 log (↓95.8) | 4.6 × 103; 3.7 log (↓58.2) | 4.6 × 103; 3.7 log (↓58.2) |
| Total Proteolytic Count, TPC | CFU/mL | 1.9 × 102; 2.3 log (↓99.3) | 2.4 × 103; 3.4 log (↓90.8) | 2.6 × 103; 3.4 log (↓90.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.; Kozik, V.; Barbusiński, K.; Sochanik, A.; Jampilek, J.; Bąk, A. Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate. Materials 2020, 13, 5017. https://doi.org/10.3390/ma13215017
Thomas M, Kozik V, Barbusiński K, Sochanik A, Jampilek J, Bąk A. Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate. Materials. 2020; 13(21):5017. https://doi.org/10.3390/ma13215017
Chicago/Turabian StyleThomas, Maciej, Violetta Kozik, Krzysztof Barbusiński, Aleksander Sochanik, Josef Jampilek, and Andrzej Bąk. 2020. "Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate" Materials 13, no. 21: 5017. https://doi.org/10.3390/ma13215017
APA StyleThomas, M., Kozik, V., Barbusiński, K., Sochanik, A., Jampilek, J., & Bąk, A. (2020). Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate. Materials, 13(21), 5017. https://doi.org/10.3390/ma13215017