How to Proceed with Asymptomatic Modular Dual Taper Hip Stems in the Case of Acetabular Revision
Abstract
:1. Introduction
2. Objectives
- 1)
- Which suggestions are given in literature how to proceed with clinically symptomatic and asymptomatic dual taper stem-neck couplings?—Status of research and appropriate diagnostic methods.
- 2)
- What are the relevant findings based on neck adapter retrievals of the Metha® dual taper CoCr/Ti alloy hip stem design?
- 3)
- How to proceed with a clinically asymptomatic dual taper modular hip stem in case of acetabular revision?—Definition of a rational decision making model as basis for a clinical recommendation.
3. Literature on Symptomatic and Asymptomatic Dual Taper Hip Stems
4. Clinical Case Presentations and Retrieval Analysis
4.1. Metha® Dual Taper CoCr/Ti Alloy Couplings with Adverse Local Tissue Reactions
4.2. Retrieval Analysis of CoCr Neck Adapters Revised for Other Reasons than Adverse Local Tissue Reactions
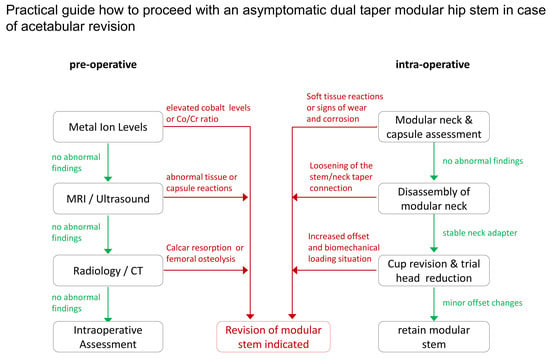
5. Decision Making Model for Asymptomatic Dual Taper Stems in Case of Acetabular Revision
6. Discussion
- 1)
- The status of research and appropriate diagnostic methods in context to clinically symptomatic and asymptomatic dual taper stem-neck couplings was evaluated based on a systematic literature review.
- 2)
- A retrieval analysis of thirteen Metha® dual taper CoCr/ Ti alloy hip stems was performed.
- 3)
- A rational decision making model as basis for a clinical recommendation was developed.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Evans, J.T.; Evans, J.P.; Walker, R.W.; Blom, A.W.; Whitehouse, M.R. Sayers A: How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-Up. Lancet 2019, 393, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Sadoghi, P.; Liebensteiner, M.; Agreiter, M.; Leithner, A.; Böhler, N.; Labek, G. Revision surgery after total joint arthroplasty: A complication-Based analysis using worldwide arthroplasty registers. J. Arthroplast. 2013, 28, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Toni, A.; Sudanese, A.; Paderni, S.; Guerra, E.; Bianchi, G.; Antonietti, B.; Giunti, A. Cementless hip arthroplasty with a modular neck. Chir. Organi. Mov. 2001, 86, 73–85. [Google Scholar]
- Traina, F.; De Fine, M.; Biondi, F.; Tassinari, E.; Galvani, A.; Toni, A. The influence of the centre of rotation on implant survival using a modular stem hip prosthesis. Int. Orthop. 2009, 33, 1513–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archibeck, M.J.; Cummins, T.; Carothers, J.; Junick, D.W.; White, R.E. A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 443–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, A.; Jung, E.; Levine, B.R. Modularity of the femoral component in total hip arthroplasty. J. Am. Acad. Orthop. Surg. 2012, 20, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Traina, F.; De Clerico, M.; Biondi, F.; Pilla, F.; Tassinari, E.; Toni, A. Sex differences in hip morphology: Is stem modularity effective for total hip replacement? J. Bone Jt. Surg. Am. 2009, 91, 121–128. [Google Scholar] [CrossRef]
- Ollivier, M.; Parratte, S.; Galland, A.; Lunebourg, A.; Flecher, X.; Argenson, J.N. Titanium-Titanium modular neck for primary THA. Result of a prospective series of 170 cemented THA with a minimum follow-Up of 5 years. Orthop. Traumatol. Surg. Res. 2015, 101, 137–142. [Google Scholar] [CrossRef]
- Traina, F.; De Fine, M.; Tassinari, E.; Sudanese, A.; Calderoni, P.P.; Toni, A. Modular neck prostheses in DDH patients: 11-Year results. J. Orthop. Sci. 2011, 16, 14–20. [Google Scholar] [CrossRef]
- Gerhardt, D.M.; Bisseling, P.; de Visser, E.; van Susante, J.L. Modular necks in primary hip arthroplasty without anatomical deformity: No clear benefit on restoration of hip geometry and dislocation rate. An exploratory study. J. Arthroplast. 2014, 29, 1553–1558. [Google Scholar] [CrossRef]
- Wittenberg, R.H.; Steffen, R. Comparative 5-Year results of short hip total hip arthroplasty with Ti- or CoCr-Neck adapters. Orthopedics 2015, 38, S33–S39. [Google Scholar] [CrossRef] [PubMed]
- Fitch, D.A.; Ancarani, C.; Bordini, B. Long-Term survivorship and complication rate comparison of a cementless modular stem and cementless fixed neck stems for primary total hip replacement. Int. Orthop. 2015, 39, 1827–1837. [Google Scholar] [CrossRef] [Green Version]
- Von Lewinski, G.; Floerkemeier, T. 10-Year experience with short stem total hip arthroplasty. Orthopedics 2015, 38, S51–S56. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, C.; Schellen, B.; Dargel, J.; Beckmann, J.; Eysel, P.; Steffen, R. Low short stem revision rates: 1-11 years results from 1888 total hip arthroplasties. J. Arthroplast. 2017, 32, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Toni, A.; Giardina, F.; Guerra, G.; Sudanese, A.; Montalti, M.; Stea, S.; Bordini, B. 3rd generation alumina-On-Alumina in modular hip prosthesis: 13 to 18 years follow-Up results. HIP Int. 2017, 27, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Grupp, T.M.; Weik, T.; Bloemer, W.; Knaebel, H.-P. Modular titanium alloy neck adapter failures in hip replacement–Failure mode analysis and influence of implant material. BMC Musculoskelet. Disord. 2010, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, J.J.; Cooper, H.J.; Urban, R.M.; Wixson, R.L.; Della Valle, C.J. What do we know about taper corrosion in total hip arthroplasty? J. Arthroplast. 2014, 29, 668–669. [Google Scholar] [CrossRef]
- Krishnan, H.; Krishnan, S.P.; Blunn, G.; Skinner, J.A.; Hart, A.J. Modular neck femoral stems. Bone Jt. J. 2013, 95, 1011–1021. [Google Scholar] [CrossRef]
- Mistry, J.B.; Chughtai, M.; Elmallah, R.K.; Diedrich, A.; Le, S.; Thomas, M.; Mont, M.A. Trunnionosis in total hip arthroplasty: A review. J. Orthopaed. Traumatol. 2016, 17, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wodecki, P.; Sabbah, D.; Kermarrec, G.; Semaan, I. New type of hip arthroplasty failure releated to modular femoral components: Breakage at the neck-stem junction. Orthop. Traumatol. Surg. Res. 2013, 99, 741–744. [Google Scholar] [CrossRef] [Green Version]
- Mihalko, W.M.; Assaf, D.; Sungu, M.T. Reproducing the hip center with a femoral neck-Retaining implant. Orthopedics 2015, 38, S21–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pour, A.E.; Borden, R.; Murayama, T.; Groll-Brown, M.; Blaha, J.D. High Risk of Failure With Bimodular Femoral Components in THA. Clin. Orthop. Relat. Res. 2016, 474, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwood, S.A.; Patten, E.W.; Bozic, K.J.; Pruitt, L.A.; Ries, M.D. Corrosion-Induced fracture of a double-Modular hip prosthesis: A case report. J. Bone Jt. Surg. Am. 2010, 92, 1522–1525. [Google Scholar] [CrossRef] [PubMed]
- Sotereanos, N.G.; Sauber, T.J.; Tupis, T.T. Modular femoral neck fracture after primary total hip arthroplasty. J. Arthroplast. 2013, 28, 196.e7–196.e9. [Google Scholar] [CrossRef] [PubMed]
- Fokter, S.K.; Rudolf, R.; Molicnik, A. Titanium alloy femoral neck fracture–Clinical and metallurgical analysis in 6 cases. Acta Orthop. 2016, 87, 198–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokter, S.K.; Molicnik, A.; Kavalar, R.; Pelicon, P.; Rudolf, R.; Gubeljak, N. Why do some titanium-Alloy total hip arthroplasty modular necks fail. J. Mech. Behav. Biomed. Mater. 2016, 69, 107–114. [Google Scholar] [CrossRef]
- Ceretti, M.; Falez, F. Modular titanium alloy neck failure in total hip replacement: Analysis of a relapse case. SICOT J. 2016, 20, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Menciere, M.L.; Amouyel, T.; Taviaux, J.; Bayle, M.; Laterza, C.; Mertl, P. Fracture of the cobalt-Chromium modular femoral neck component in total hip arthroplasty. Orthop. Traumatol. Surg. Res. 2014, 100, 565–568. [Google Scholar] [CrossRef] [Green Version]
- Murena, L.; Maritan, G.; Concina, C.; Scamacca, V.; Ratti, C.; Canton, G. Fracture of cobalt-Crome modular neck in total hip arthroplasty. Acta Biomed. 2019, 90 (Suppl. 12), 187–191. [Google Scholar]
- Kovac, S.; Mavcic, B.; Kotnik, M.; Levasic, V.; Sirse, M.; Fokter, S. What Factors Are Associated with Neck Fracture in One Commonly Used Bimodular THA Design? A Multicenter, Nationwide Study in Slovenia. Clin. Orthop. Relat. Res. 2019, 466, 1324–1332. [Google Scholar] [CrossRef]
- Meftah, M.; Halleem, A.M.; Burn, M.B.; Smith, K.M.; Incavo, S.J. Early Corrosion-Relared Failure of the Rejuvenate Modular Total Hip Replacement. J. Bone Jt. Surg. Am. 2014, 96, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martino, I.; Assini, J.B.; Elpers, M.E.; Wright, T.M.; Westrich, G.H. Corrosion and Fretting of a Modular Hip System: A Retrieval Analysis of 60 Rejuvenate Stems. J. Arthroplast. 2015, 30, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, D.; Liow, M.H.; Tsai, T.Y.; Leone, W.A.; Li, G.; Kwon, Y.M. Early Outcome of Revision Surgery for Taper Corrosion of Dual Taper Total Hip Arthroplasty in 187 Patients. J. Arthroplast. 2016, 31, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Kolatat, K.; Perino, G.; Wilner, G.; Kaplowitz, E.; Ricciardi, B.F.; Boettner, F.; Westrich, G.H.; Jerabek, S.A.; Goldring, S.R.; Purdue, P.E. Adverse local tissue reaction (ALTR) associated with corrosion products in metal-On-Metal and dual modular neck total hip replacements is associated with upregulation of interferon gamma-Mediated chemokine signaling. J. Orthop. Res. 2015, 33, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.M.; Khormaee, S.; Liow, M.H.; Tsai, T.Y.; Freiberg, A.A.; Rubash, H.E. Asymptomatic Pseudo-Tumors in Patients with Taper Corrosion of a Dual-Taper Modular Femoral Stem: MARS-MRI and Metal Ion Study. J. Bone Jt. Surg. 2016, 98, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.M.; Tsai, T.Y.; Leone, W.A.; Liow, M.H. Sensitivity and Specificity of Metal Ion Levels in Predicting “Pseudo-tumors” due to Taper Corrosion in Patients with Dual Taper Modular Total Hip Arthroplasty. J. Arthroplast. 2017, 32, 996–1000. [Google Scholar] [CrossRef]
- Omlor, G.W.; Kretzer, J.P.; Reinders, J.; Streit, M.R.; Bruckner, T.; Gotterbarm, T.; Aldinger, P.R.; Merle, C. In vivo serum titanium ion levels following modular neck total hip arthroplasty–10 years results in 67 patients. Acta Biomater. 2013, 9, 6278–6782. [Google Scholar] [CrossRef]
- Gofton, W.; Beaule, P.E. Serum Metal Ions with a Titanium Modular Neck Total Hip Replacement System. J. Arthroplast. 2015, 30, 1781–1786. [Google Scholar] [CrossRef]
- Vundelinckx, B.J.; Verhelst, L.A.; De Schepper, J. Taper Corrosion in Modular Hip Prostheses Analysis of Serum Metal Ions in 19 Patients. J. Arthroplast. 2013, 28, 1218–1223. [Google Scholar] [CrossRef]
- Cooper, H.J.; Urban, R.M.; Wixson, R.L.; Meneghini, R.M.; Jacobs, J.J. Adverse Local Tissue Reaction arising from Corrosion at the Femoral Neck-Body Junction in a Dual-Taper Stem with a Cobalt-Chromium Modular Neck. J. Bone Jt. Surg. 2013, 95, 865–872. [Google Scholar] [CrossRef]
- Cheung, A.C.; Banerjee, S.; Cherian, J.J.; Wong, F.; Butany, J.; Gilbert, C.; Overgaard, C.; Syed, K.; Zywiel, M.G.; Jacobs, J.J.; et al. Systemic cobalt toxicity from total hip arthroplasties. Bone Jt. J. 2016, 98, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molloy, D.O.; Munir, S.; Jack, C.M.; Cross, M.B.; Walter Wl Walter, W.K. Fretting and Corrosion in Modular-Neck total Hip Arthroplasty Femoral Stems. J. Bone Jt. Surg. Am. 2014, 96, 488–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, E.; Ward, D.M.; Robbins, C.E.; Nandi, S.; Bono, J.V.; Talmo, C.T. Corrosion and Adverse Local Tissue Reaction in One Type of Modular Neck Stem. J. Arthroplast. 2015, 30, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.T.; Mefath, M.; Paranilam, J.; Incavo, S.J. Eighty-Six Percent Failure Rate of a Modular-Neck Femoral Stem Design at 3 to 5 Years: Lessons Learned. J. Bone Jt. Surg. 2016, 98, e491–e497. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.P.S.; Webb, J.; Sloan, K.; Beaver, R.J. Corrosion at the neck-stem junction as a cause of metal ion release and pseudotumour formation. J. Bone Jt. Surg. 2012, 94, 895–900. [Google Scholar] [CrossRef]
- Kop, A.M.; Keogh, C.; Swarts, E. Proximal component modularity in THA–At what cost? An implant retrieval study. Clin. Orthop. Relat. Res. 2012, 470, 1885–1894. [Google Scholar] [CrossRef] [Green Version]
- Wooster, B.M.; Lachiewicz, P.F. Trunnionosis: Is it really a problem? Semin. Arthroplast. 2017, 28, 206–210. [Google Scholar] [CrossRef]
- Walsh, C.P.; Hubbard, J.C.; Nessler, J.P.; Markel, D.C. Revision of recalled modular neck Rejuvenate and ABG femoral implants. J. Arthroplast. 2015, 30, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Barlow, B.T.; Assini, J.; Boles, J.; Lee, Y.Y.; Westrich, G.H. Short-Term metal ion trends following removal of recalled modular neck femoral stems. J. Arthroplast. 2015, 30, 1191–1196. [Google Scholar] [CrossRef]
- Silverton, C.D.; Jacobs, J.J.; Devitt, J.W.; Cooper, H.J. Midterm results of a femoral stem with a modular neck design: Clinical outcomes and metal ion analysis. J. Arthroplast. 2014, 29, 1768–1773. [Google Scholar] [CrossRef]
- Lanting, B.A.; Teeter, M.G.; Vasarhelyi, E.M.; Ivanov, T.G.; Howard, J.L.; Naudie, D.D.R. Correlation of corrosion and biomechanics in the retrieval of a single modular neck total hip arthroplasty design: Modular neck total hip arthroplasty system. J. Arthroplast. 2015, 30, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.; Ross, D.; Restrepo, S.; Heller, S.; Goyal, N.; Moore, R.; Hozack, W.J. Adverse clinical outcomes in a primary modular neck/stem system. J. Arthroplast. 2014, 29, S173–S178. [Google Scholar] [CrossRef] [PubMed]
- Chillemi, M.; Placella, G.; Caraffa, A.; Cerulli, G.; Antinolfi, P. Serologic and radiographic outcome of total hip arthroplasty with CoCr modular neck at mid-term follow-Up. Musculoskelet. Surg. 2017, 101, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Liow, M.H.L.; Urish, K.L.; Preffer, F.I.; Nielson, G.P.; Kwon, Y.M. Metal Ion Levels Are Not Correlated with Histopathology of Adverse Local Tissue Reactions in Taper Corrosion of Total Hip Arthroplasty. J. Arthroplast. 2016, 31, 1797–1802. [Google Scholar] [CrossRef]
- Barlow, B.T.; Ortiz, P.A.; Fiels, K.G.; Burge, A.J.; Potter, H.G.; Westrich, G.H. Magnetic resonance imaging predicts adverse local tissue reaction histologic severity in modular neck total hip arthroplasty. J. Arthroplast. 2016, 31, 2325–2331. [Google Scholar] [CrossRef]
- Hussey, D.K.; McGrory, B.J. Ten-Year cross-Sectional study of mechanically assisted crevice corrosion in 1352 consecutive patients with metal-On-Polyethylene total hip arthroplasty. J. Arthroplast. 2017, 32, 2546–2551. [Google Scholar] [CrossRef]
- Canham, C.D.; Muradov, P.I.; Simpson, J.B.; Incavo, S.J. Corrosion and adverse local tissue reaction after total hip arthroplasty with a modular titanium alloy femoral neck. Arthoplasty Today 2017, 3, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.M.; Fehring, T.K.; Lombardi, A.V.; Barnes, C.L.; Cabanela, M.E.; Jacobs, J.J. Risk stratification algorithm for management of patients with dual modular taper total hip arthroplasty: Consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons and the Hip Society. J. Arthroplast. 2014, 29, 2060–2064. [Google Scholar] [CrossRef]
- Kwon, Y.M. Evaluation of the Painful Dual Taper Modular Neck Stem Total Hip Arthroplasty: Do They All Require Revision? J. Arthroplast. 2016, 31, 1385–1389. [Google Scholar] [CrossRef]
- Xia, Z.; Ricciardi, B.F.; Liu, Z.; von Ruhland, C.; Ward, M.; Lord, A.; Hughes, L.; Goldring, S.R.; Purdue, E.; Murray, D.; et al. Nano-Analysis of wear particles from metal-On-Metal and non metal-On-Metal dual modular neck hip arthroplasty. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1205–1217. [Google Scholar] [CrossRef]
- Perino, G.; Ricciardi, B.F.; Jerabek, S.A.; Martignoni, G.; Wilner, G.; Maass, D.; Goldring, S.R.; Purdue, P.E. Implant based differences in adverse local tissue reaction in failed total hip arthroplasties: A morphological and immunohistochemical study. BMC Clin. Pathol. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawietz, L.; Classen, R.; Schröder, J.H.; Dynybil, C.; Perka, C.; Skwara, A.; Neidel, A.; Gehrke, T.; Frommelt, L.; Hansen, T.; et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J. Clin. Pathol. 2006, 59, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenn, V.; Morawietz, L.; Burmester, G.R.; Kinne, R.W.; Mueller-Ladner, U.; Mueller, B.; Haupl, T. Synovitis score: Discrimination between chronic low-grade and high-Grade synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Buente, D.; Huber, G.; Bishop, N.; Morlock, M. Quantification of material loss from neck piece taper junctions of a bimodular primary hip prosthesis. A retrieval study from 27 failed Rejuvenate bimodular hip arthroplasties. Bone Jt. J. 2015, 97, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.A.; Della Valle, C.J.; Bohl, D.D.; Kelly, M.P.; Hall, D.J.; Pourzal, R.; Jacobs, J.J. Serum metal levels for diagnosis of adverse local tissue reactions secondary to corrosion in metal-On-Polyethylene total hip arthroplasty. J. Arthroplast. 2017, 32, S272–S277. [Google Scholar] [CrossRef] [PubMed]
- Nawabi, D.H.; Do, H.T.; Ruel, A.; Lurie, B.; Elpers, M.E.; Wright, T.; Potter, H.G.; Westrich, G.H. Comprehensive analysis of a recalled modular total hip system and recommendations for management. J. Bone Jt. Surg. 2016, 98, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretzer, J.P.; Jakubowitz, E.; Krachler, M.; Thomsen, M.; Heisel, C. Metal release and corrosion effects of modular neck total hip artrhroplasty. Int. Orthop. 2009, 33, 1531–1536. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.M.; MacAuliffe, J.; Arauz, P.G.; Peng, Y. Sensitivity and specificity of metal ion level in predicting adverse local tissue reactions due to head-Neck taper corrosion in primary metal-On-Polyethylene total hip arthroplasty. J. Arthroplast. 2018. [Google Scholar] [CrossRef]
- Della Valle, C.J.; Calkins, T.E.; Jacobs, J.J. Diagnosing Taper Corrosion: When is it the taper and when is it something else? J. Arthroplast. 2018, 33. [Google Scholar] [CrossRef]
- McGrory, B.J.; Payson, A.M.; MacKenzie, J.A. Elevated intra-Articular cobalt and chromium levels in mechanically assisted crevice corrosion in metal-on-polyethylene total hip arthroplasty. J. Arthroplast. 2017, 32, 1654–1658. [Google Scholar] [CrossRef]
- Elmallah, R.K.; Cherian, J.J.; Meneghini, R.M.; Hozack, W.J.; Westrich, G.H.; Mont, M.A. How to approach a recalled dual modular hip implant: An update. J. Arthroplast. 2016, 31, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Morozov, P.P.; Sana, M.; McGrory, B.J.; Farraher, S.W.; Abrahams, T.G. Comparison of Pre-Revision Magnetic Resonance Imaging and Operative Findings in Mechanically Assisted Crevice Corrosion in Symptomatic Metal-On-Polyethylene Total Hip Arthroplasties. J. Arthroplast. 2017, 32, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Burge, A.J.; Gold, S.L.; Lurie, B.; Nawabi, D.H.; Fields, K.G.; Koff, M.F.; Westrich, G.; Potter, H.G. MR imaging of adverse local tissue reactions around rejuvenate modular dual-Taper stems. Radiology 2015, 277, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Koff, M.F.; Esposito, C.; Shah, P.; Miranda, M.; Baral, E.; Fields, K.; Bauer, T.; Padgett, D.E.; Wright, T.; Potter, H.G. MRI of THA Correlates with Implant Wear and Tissue Reactions: A Cross-Sectional Study. Clin. Orthop. Relat. Res. 2019, 477, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.P.; Hubbard, J.C.; Nessler, J.P.; Markel, D.C. MRI Findings Associated with Recalled Modular Femoral Neck Rejuvenate and ABG Implants. J. Arthroplast. 2015, 30, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Frisch, N.B.; Wessell, N.M.; Taliaferro, K.; van Holsbeeck, M.; Silverton, C.D. Ultrasound findings in asymptomatic patients with modular metal on metal total hip arthroplasty. Skelet. Radiol. 2017, 46, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Ebramzadeh, E.; Nelson, S.; Takamura, K.; DeSmet, K.; Amstutz, H.C. Histological features of pseudo-Tumor-Like tissues from metal-On-Metal hips. Clin. Orthop. Relat. Res. 2010, 468, 2321–2327. [Google Scholar] [CrossRef] [Green Version]
- Werner, S.D.; Bono, J.V.; Nandi, S.; Ward, D.M.; Talmo, C.T. Adverse tissue reactions in modular exchangeable neck implants: A report of two cases. J. Arthroplast. 2013, 28, 543–545. [Google Scholar] [CrossRef]
- Baxmann, M.; Pfaff, A.; Schilling, C.; Grupp, T.M.; Morlock, M.M. Biomechanical Evaluation of the Fatigue Performance, the Taper Corrosion and the Metal Ion Release of a Dual Taper Hip Prosthesis under Physiological Environmental Conditions. Biotribology 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Sporer, S.M. How to do a revision total hip arthroplasty. Revision of the acetabulum. Instr. Course Lect. 2012, 61, 303–311. [Google Scholar] [CrossRef]
- Tohtz, S.W.; Sassy, D.; Matziolis, G.; Preininger, B.; Perka, C.; Hasart, O. CT evaluation of native acetabular orientation and localization: Sex-Specific data comparison on 336 hip joints. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2010, 18, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Skendzel, J.G.; Blaha, J.D.; Urquhart, A.G. Total hip arthroplasty modular neck failure. J. Arthroplast. 2011, 26, 338.e1–338.e4. [Google Scholar] [CrossRef] [PubMed]
- Traina, F.; Baleani, M.; Erani, P.; Bordini, B.; de Fine, M.; Stea, S.; Toni, A. Failure of Modular Necks in Primary Total Hip Replacement: Can We Prevent It? In Proceedings of the Scientific Exhibit 79th American Academy of Orthopaedic Surgeons, San Francisco, CA, USA, 14–16 April 2012.
- Benazzo, F.; Perticarini, L. Comment on: Modular titanium alloy neck failure in total hip replacement. SICOT J. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buente, D.; Bryant, M.; Ward, M.; Neville, A.; Morlock, M.; Huber, G. The taper corrosion pattern observed for one bi-modular stem design is related to geometry-Determined taper mechanics. Med Eng. Phys. 2017, 46, 79–88. [Google Scholar] [CrossRef]
- Cafri, G.; Graves, S.E.; Sedrakyan, A.; Fan, J.; Calhoun, P.; de Steiger, R.N.; Cuthbert, A.; Lorimer, M.; Paxton, E.W. Postmarket surveillance of arthroplasty device components using machine learning methods. Pharmacoepidemiol. Drug Saf. 2019, 28, 1440–1447. [Google Scholar] [CrossRef]
- Koziara, C.R.; Lombardo, D.J.; Petersen-Fitts, G.R.; Jildeh, T.R.; Morawa, L. Effects of cobalt and chromium levels following modular hip stem total hip arthroplasty. Orthopedics 2016, 39, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.; Kiss, M.O.; Massé, V.; Lavigne, M.; Matta, J.; Venditolli, P.A. Effect of femoral stem modular neck’s material on metal ion release. Open Orthop. J. 2017, 11, 1337–1344. [Google Scholar] [CrossRef]
- Laurencon, J.; Augsburger, M.; Faouzi, M.; Becce, F.; Hassani, H.; Rüdiger, H.A. Systemic metal ion levels in patients with modular neck stems: A Prospective cohort study. J. Arthroplast. 2016, 31, 1750–1755. [Google Scholar] [CrossRef]
- Colas, S.; Allalou, A.; Poichotte a Piriou, P.; Dray-Spira, R.; Zureik, M. Exchangeable femoral neck (dual modular) THA prostheses have poorer survivorship than other designs: A nationwide cohort of 324,108 patients. Clin. Orthop. Relat. Res. 2017, 475, 2046–2059. [Google Scholar] [CrossRef] [Green Version]
- Graves, S.; de Steiger, R.; Lewis, P.; Harris, I. Australian Orthopaedic Association National Joint Replacement Registry for Hip, Knee & Shoulder Arthroplasty. Annu. Rep. 2018, 155–156. [Google Scholar]
- Mihalko, W.M.; Wimmer, M.A.; Pacione, C.A.; Laurent, M.P.; Murphy, R.F.; Rider, C. How Have Alternative Bearings and Modularity Affected Revision Rates in Total Hip Arthroplasty? Clin. Orthop. Relat. Res. 2014, 472, 3747–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, S.E.; De Steiger, R.; Davidson, D.; Donnelly, W.; Rainbird, S.; Lorimer, M.F.; Cashman, K.S.; Vial, R. The use of femoral stems with exchangeable necks in primary total hip arthroplasty increases the rate of revision. Bone Jt. J. 2017, 99, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Su, S.L.; Koch, C.N.; Nguyen, T.M.; Burket, J.C.; Wright, T.M.; Westrich, G.H. Retrieval analysis of neck-stem coupling in modular hip prostheses. J. Arthroplast. 2017, 32, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Somers, J.F.A.; Dedrye, L.; Goeminne, S. Metal ion levels in ceramic-On-Ceramic THR with cobalt-Chrome modular necks: Analysis of cobalt and chromium serum levels in 23 healthy hip patients. Hip Int. 2017, 27, 21–25. [Google Scholar] [CrossRef] [PubMed]







| Study | Prosthesis | Neck/ Stem Material | Mean Age (Years) | Follow-up (Years) | Number of Patients | Study Group | Serum Level (µg/L) | Co/Cr Ratio | MRI Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Co | Cr | Ti | |||||||||
| Omlor et al. 2013 [37] | Profemur E | Ti6Al4V/Ti6Al4V | 66.0 | 9.0 | 67 | Modular (Uni-/bilateral) | - | - | 3.0/6.0 | - | - |
| CLS | Ti6Al4V | 71.0 | 11 | Non-modular (Uni-/bilateral) | - | - | 2.7/6.2 | - | - | ||
| Molloy et al. 2014 [42] | ABGII | Vitallium/TMZF | 64.3 | 3.4 3.6 | 7 8 | Revised Non-revised | 6.3 3.9 | 1.3 1.1 | - - | 6.4 3.7 | 86% 13% |
| Silverton et al. 2014 [50] | Profemur Z | Ti6Al4V/Ti6Al4V | 59.5 | 4.5 | 137 | Follow-up | 2.4 | 1.7 | 4.2 | - | - |
| Gofton et al. 2015 [38] | Profemur TL | Ti6Al4V/Ti6Al4V | 60.2 | 2.0 | 25 25 | MoM articulation MoP articulation | 2.5 0.3 | 2.1 0.3 | 2.8 2.9 | - - | - - |
| Lanting et al. 2015 [51] | Rejuvenate | Vitallium/TMZF | 65.0 | 1.7 | 19 | Revised | 5.5 | 0.8 | 3.0 | 8.2 | 83% |
| Restrepo et al. 2014 [52] | ABGII | Vitallium/TMZF | 61.0 | 2.0 | 85 110 | Symptomatic Asymptomatic | 4.0 3.4 | 1.2 1.2 | - - | 46% 11% | |
| Barlow et al. 2016 [55] | Rejuvenate | Vitallium/TMZF | 65.8 | 2.4 | 90 | Revised | 6.6 | 1.3 | - | - | 81% |
| Chillemi et al. 2017 [53] | ABGII | Vitallium/TMZF | 69.4 65.1 | >1.0 | 5 17 | Symptomatic Asymptomatic | 3.0 3.6 | 0.4 0.7 | - - | 7.4 9.6 | - - |
| Kwon et al. 2017 [36] | Rejuvenate/ABGII | Vitallium/TMZF | 59.0 | 1.4 | 90 | Pseudotumor | 5.0 | 0.8 | - | 6.0 | 100% |
| 58.0 | 1.3 | 58 | No pseudotumor | 3.7 | 0.8 | - | 3.7 | 0 % | |||
| Liow et al. 2016 [54] | Rejuvenate/ABGII | Vitallium/TMZF | 57.4 | 2.3 | 31 | Revised | 3.8 | 1.0 | - | 3.8 | 100 % |
| Patient (n) | Indication & Revision Procedure | Time In Situ (mths) | Sex | Age (years) | BMI (kg/m2) | Stem Size & Side | CCD-Angle Version | Head Size & Offset | Head Material | Serum Level (µg/L) | Co/Cr Ratio | Max. Wear Depth (µm) | Material Loss (mm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Soft tissue reactions progressive pain & swelling, ALTR & pseudo-tumor formation | 61 | Female | 60 | - | 2 right | 135° 0° | 40 +0 | Ceramic/Ti-Sleeve | Co=31.3 Cr=0.3 | R=104 | 37 | 2.6 |
| 2 | Groin pain, hip aspiration—cloudy-yellow synovial fluid, local tissue infiltrations, thickened capsular tissue | 84 | Female | 67 | - | 3 left | 135° 0° | 36 −4.0 | Ceramic | - | - | 72 | 5.0 |
| 3 | Soft tissue reactions, persistent hip pain ALTR, positive MRI findings, peri-prosthetic joint infection, extensive debridement of capsular tissue & metal debris | 94 | Female | 76 | 32.0 | 2 left | 130° 7.5° AV | 32 +4.0 | Ceramic | Co=8.3 Cr=0.8 | R=10.37 | 82 | 6.5 |
| 4 | Soft tissue reactions, persistent hip pain & swelling, elevated Co level, pseudo-tumor resection & debridement of metal debris, severe loosening of ScrewCup® SC | 96 | Female | 58 | 24.6 | 2 - | 135° 0° | 32 −4.0 | Ceramic | - | - | 128 | 9.7 |
| 5 | CT hip scan, migrated Hofer cup, suspicion cup loosening, exploration oft he hip, accumulation of metal debris, muddy-yellow fluid accumulation within joint capsula | 128 | Female | 79 | - | 2 left | 135° 7.5°AV | 36 +0 | Ceramic | - | - | 100 | 12.0 |
| Patient (n) | Revision | Time In Situ (mths) | Sex | Age (Years) | BMI (kg/m2) | Stem Size & Side | CCD-Angle Version | Head Size (mm) | Head Offset (mm) | Head Material | Max. Wear Depth (µm) | Material Loss (mm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Osseointegration insufficient | 13 | Male | 73 | 29.7 | 5 right | 130° 0° | 36 | +4.0 | Ceramic | 7 | 0.1 |
| 2 | Malposition cup | 26 | Female | 77 | 31.6 | 1 right | 130° 0° | 32 | −4.0 | Ceramic | 16 | 0.5 |
| 3 | Patient discomfort | 36 | Female | 63 | 20.2 | 2 right | 135° 0° | 32 | −4.0 | Ceramic | 78 | 5.1 |
| 4 | Cup loosening | 54 | Female | 69 | - | 2 left | 135° 0° | 36 | −4.0 | Ceramic | 39 | 3.0 |
| 5 | Patient discomfort | 72 | Female | 67 | - | 3 right | 130° 0° | 32 | Ceramic/Ti-Sleeve | 86 | 6.3 | |
| 6 | Luxation | 80 | Female | 63 | 29.1 | 3 left | 130° 0° | 32 | −4.0 | Ceramic | 61 | 5.3 |
| 7 | Acetabulum fracture | 104 | Female | 63 | 25.2 | 2 right | 135° 0° | 28 | +5.0 | Ceramic | 90 | 5.5 |
| 8 | Periprosthetic fracture | 111 | Female | 74 | - | 3 right | 135° 7.5° RV | 28 | +3.5 | Ceramic | 14 | 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grupp, T.M.; Baxmann, M.; Jansson, V.; Windhagen, H.; Heller, K.-D.; Morlock, M.M.; Knaebel, H.-P. How to Proceed with Asymptomatic Modular Dual Taper Hip Stems in the Case of Acetabular Revision. Materials 2020, 13, 1098. https://doi.org/10.3390/ma13051098
Grupp TM, Baxmann M, Jansson V, Windhagen H, Heller K-D, Morlock MM, Knaebel H-P. How to Proceed with Asymptomatic Modular Dual Taper Hip Stems in the Case of Acetabular Revision. Materials. 2020; 13(5):1098. https://doi.org/10.3390/ma13051098
Chicago/Turabian StyleGrupp, Thomas M., Marc Baxmann, Volkmar Jansson, Henning Windhagen, Karl-Dieter Heller, Michael M. Morlock, and Hanns-Peter Knaebel. 2020. "How to Proceed with Asymptomatic Modular Dual Taper Hip Stems in the Case of Acetabular Revision" Materials 13, no. 5: 1098. https://doi.org/10.3390/ma13051098
APA StyleGrupp, T. M., Baxmann, M., Jansson, V., Windhagen, H., Heller, K. -D., Morlock, M. M., & Knaebel, H. -P. (2020). How to Proceed with Asymptomatic Modular Dual Taper Hip Stems in the Case of Acetabular Revision. Materials, 13(5), 1098. https://doi.org/10.3390/ma13051098