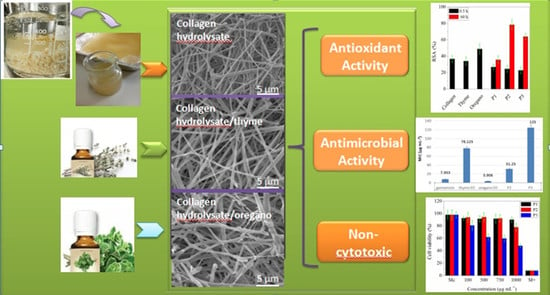
Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Preparation and Characterisation of Concentrated Collagen Hydrolysate
2.3. Essential Oils (EOs)
2.4. Preparation of Electrospun Collagen Nanofibres Loaded with Essential Oils
2.5. Investigation Methods
2.5.1. Loaded Efficiency (LE) of Essential Oils
2.5.2. Determination of Total Phenolic Content (TPC)
2.5.3. DPPH Radical-Scavenging Assay
2.5.4. ATR-FTIR Spectroscopy
2.5.5. Scanning Electron Microscopy (SEM) with Energy Dispersive X-Ray Analysis (EDX)
2.5.6. Biocompatibility Assay
2.5.7. Determination of Antimicrobial Activity
2.5.8. Statistical Analysis
3. Results
3.1. Characterization of Concentrated Collagen Hydrolysate
3.2. Efficiency of Essential Oils Loading
3.3. Total Phenolic Content (TPC)
3.4. DPPH Radical Scavenging Activity Assessment
3.5. Structural Analysis by ATR-FTIR
3.6. SEM/EDX Analyses
3.7. In Vitro Evaluation of Collagen and Collagen Loaded with Essential Oils Nanofibres Biocompatibility
3.8. Antimicrobial Activity
3.8.1. The Qualitative Screening of the Antimicrobial Properties
3.8.2. The Quantitative Assay Results
3.8.3. Minimal Concentration for Biofilm Eradication (MCBE)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leidy, R.; Ximena, Q.-C.M. Use of electrospinning technique to produce nanofibres for food industries: A perspective from regulations to characterizations. Trends Food Sci. Technol. 2019, 85, 92–106. [Google Scholar] [CrossRef]
- Uyar, T.; Kny, E. Electrospun Materials for Tissue Engineering and Biomedical Applications: Research, Design and Commercialization; Elsevier: Amsterdam, The Netherlands; Woodhead Publishing: Sawston, Cambridge, UK, 2017. [Google Scholar]
- Fahami, A.; Fathi, M. Development of cress seed mucilage/PVA nanofibers as a novel carrier for vitamin A delivery. Food Hydrocoll. 2018, 81, 31–38. [Google Scholar] [CrossRef]
- Aliheidari, N.; Aliahamad, N.; Agarwal, M.; Dalir, H. Electrospun Nanofiber for Label-Free Sensor Applications. Sensors 2019, 19, 3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimin, L.; Jinsong, D.; Xingxing, J.; Pengfei, L.; Xuefeng, J.; Jiang, Y. Polyurethane/Keratin/AgNPs nanofibrous mats as catalyst support for 4-nitroaniline reduction. Mater. Lett. 2019, 237, 9–13. [Google Scholar]
- McKee, M.G.; Layman, J.M.; Cashion, M.P.; Long, T.E. Phospholipid nonwoven electrospun membranes. Science 2006, 311, 353–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashion, M.P.; Li, X.; Geng, Y.; Hunley, M.T.; Long, T.E. Gemini surfactant electrospun membranes. Langmuir 2010, 26, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Electrospinning of nanocomposite nanofibers from cyclodextrin and Laponite. Compos. Commun. 2019, 12, 33–38. [Google Scholar] [CrossRef]
- Allais, M.; Mailley, D.; Hebraud, P.; Ihiawakrim, D.; Ball, V.; Meyer, F.; Hebraud, A.; Schlatter, G. Polymer-free electrospinning of tannic acid and cross-linking in water for hybrid supramolecular nanofibres. Nanoscale 2018, 10, 9164–9173. [Google Scholar] [CrossRef]
- Fang, X.; Ma, H.; Xiao, S.; Shen, M.; Guo, R.; Cao, X.; Shi, X. Imidazolium ionic liquid-modified fibrous silica microspheres loaded with gold nanoparticles and their enhanced catalytic activity and reusability for the reduction of 4-nitrophenol. J. Mater. Chem. 2011, 21, 4493–4501. [Google Scholar] [CrossRef]
- Farkas, B.; Balogh, A.; Cselkó, R.; Molnár, K.; Farkas, A.; Borbás, E.; Marosi, G.; Nagy, Z.K. Corona alternating current electrospinning: A combined approach for increasing the productivity of electrospinning. Int. J. Pharm. 2019, 561, 219–227. [Google Scholar] [CrossRef]
- Russo, N.; Cassinelli, C.; Torre, E.; Morra, M.; Iviglia, G. Improvement of the Physical Properties of Guided Bone Regeneration Membrane from Porcine Pericardium by Polyphenols-Rich Pomace Extract. Materials 2019, 12, 2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panduranga, K.R. Recent developments of collagen-based materials for medical applications and drug delivery systems. J. Biomater. Sci. Polym. Ed. 1996, 7, 623–645. [Google Scholar] [CrossRef] [PubMed]
- Rapa, M.; Darie-Nita, R.N.; Preda, P.; Coroiu, V.; Tatia, R.; Vasile, C.; Matei, E.; Predescu, A.M.; Maxim, M.E. PLA/collagen hydrolysate/silver nanoparticles bionanocomposites for potential antimicrobial urinary drains. Polym. Plast. Technol. Mater. 2019, 58, 2041–2055. [Google Scholar] [CrossRef]
- Kumar, G.P.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. Part B 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Yorgancioglu, A.; Bayramoglu, E.E. Production of cosmetic purpose collagen containing antimicrobial emulsion with certain essential oils. Ind. Crop. Prod. 2013, 44, 378–382. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciu, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some mediterranean essential oils on human health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.D.; Guimarães, A.G. Effects of Carvacrol, Thymol and essential oils containing such monoterpenes on wound healing: A systematic review. J. Pharm. Farmacol. 2019, 71, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Azarifar, M.; Ghanbarzadeh, B.; Khiabani, M.S.; Basti, A.A.; Abdulkhani, A.; Noshirvani, N.; Hosseini, M. The optimization of gelatin-CMC based active films containing chitin nanofiber and Trachyspermum ammi essential oil by response surface methodology. Carbohydr. Polym. 2019, 208, 457–468. [Google Scholar] [CrossRef]
- Pereira dos Santos, E.; Nicácio, P.H.M.; Barbosa, F.C.; da Silva, H.N.; Andrade, A.L.S.; Fook, M.V.L.; Silva, S.M.L.; Leite, I.F. Chitosan/essential oils formulations for potential use as wound dressing: Physical and antimicrobial properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munteanu, B.S.; Sacarescu, L.; Vasiliu, A.L.; Hitruc, G.E.; Pricope, G.M.; Sivertsvik, M.; Rosnes, J.T.; Vasile, C. Antioxidant/antibacterial electrospun nanocoatings applied onto PLA films. Materials 2018, 11, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of active compounds of essential oils in Antimicrobial food packaging using a chemometric method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef] [Green Version]
- Peiwen, W.; Mele, E. Effect of antibacterial plant extracts on the morphology of electrospun poly(lactic acid) fibres. Materials 2018, 6, 923. [Google Scholar] [CrossRef] [Green Version]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Adomavicinte, E.; Jankauskaite, V.; Barsteigiene, M.M.Z.; Bernatoniene, J. Formation and investigation of electrospun Eudragit E100/oregano mats. Molecules 2019, 24, 628. [Google Scholar] [CrossRef] [Green Version]
- Gaidau, C.; Ghiga, M.; Stepan, E.; Taloi, D.; Filipescu, L. Additives and advanced biomaterials obtained from leather industry by-products. Rev. Chim. 2009, 60, 501–507. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Chirila, C.; Deselnicu, V.; Berechet, M.D. Footwear protection against fungi using thyme essential oil. Revista De Pielărie Încălţăminte 2017, 17, 173–178. [Google Scholar] [CrossRef]
- Berechet, M.D.; Chirila, C.; Deselnicu, V. Antifungal activity of some essential oils on cotton fabrics. In Proceedings of the 6th International Conference on Advanced Materials and Systems-ICAMS 2016, Bucuresti, Romania, 20–22 October 2016; pp. 197–202. [Google Scholar]
- Husnu, K.; Baser, C. Biological and Pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3120. [Google Scholar]
- Klimaszewska, E.; Seweryn, A.; Ogorzałek, M.; Nizioł–Łukaszewska, Z.; Wasilewski, T. Reduction of irritation potential caused by anionic surfactants in the use of various forms of collagen derived from marine sources in cosmetics for children. Tenside Surf. Det. 2019, 56, 180–187. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Hossein Goli, S.A.; Fathi, M. Fabrication and characterization of electrospun gelatin nanofibers crosslinked with oxidized phenolic compounds. Int. J. Biol. Macromol. 2017, 103, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Georgiev, M.; Ortan, A.; Fierascu, R.C.; Avramescu, S.M.; Ionescu, D.; Sutan, A.; Brinzan, A.; Ditu, L.M. Phyto-mediated metallic nano-architectures via Melissa officinalis L: Synthesis, characterization and biological properties. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Telcian, A.; Hussien, M.D.; Chifiriuc, M.C.; Bleotu, C.; Holban, A.M.; Curuțiu, C.; Grosu, E.; Ficai, A.; Mihăescu, G.; Grigore, R.; et al. Assessment of the anti-biofilm activity and biocompatibility of novel PE and PVC polymers. Rom. Biotechnol. Lett. 2017, 22, 12997–13004. [Google Scholar]
- Clinical & Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical & Laboratory Standards Institute: Wayne, NJ, USA, 2019. [Google Scholar]
- Fierascu, I.; Fierascu, R.C.; Somoghi, R.; Ion, R.M.; Moantă, A.; Avramescu, S.M.; Damian, C.M.; Dițu, L.M. Tuned apatitic materials: Synthesis, characterization and potential antimicrobial applications. Appl. Surf. Sci. 2018, 438, 127–135. [Google Scholar] [CrossRef]
- Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2747972.htm (accessed on 30 October 2019).
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in Vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Nagarajan, J.; Soussan, L.; Bechelany, H.; Teyssier, L.; Cavailles, V.; Pochat-Bohatier, C.; Miele, P.; Kalkura, N.; Janot, J.-M.; Balme, S. Novel biocompatible electrospun gelatin fibers mat with antibiotic drug delivery properties. J. Mater. Chem. 2015. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Ghelejlu, S.B.; Esmaiili, M.; Almasi, H. Characterization of chitosan–nanoclay bionanocomposite active films containing milk thistle extract. Int. J. Biol. Macromol. 2016, 86, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Partheniadis, I.; Karakasidou, P.; Vergkizi, S.; Nikolakakis, I. Spectroscopic examination and release of microencapsulated oregano essential oil. ADMET & DMPK 2017, 5, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Baranska, M.; Schulz, H.; Kruger, H.; Quilitzsch, R. Chemotaxonomy of aromatic plants of the genus Origanum via vibrational spectroscopy. Anal. Bioanal. Chem. 2005, 381, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Ouedrhin, W.; Balouiri, M.; Bouhdid, S.; Moja, S.; Chahdi, F.O.; Taleb, M.; Greche, H. Mixture of Origanum compactum, Origanum majorana and Thymus serpyllum essential oils: Optimization of their antibacterial effect. Ind. Crop. Prod. 2016, 89, 1–9. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basics of bacterial outer membrane permeability revised. Microbiol. Mol. Biol. Rev. 2003, 4, 593–656. [Google Scholar] [CrossRef] [Green Version]
- Avantes, S.M.; Picarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and pharmacological properties of essential oils Calamintha nepeta, Origanum virens and Thymus mastichna of Alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test. 2017, 63, 360–366. [Google Scholar] [CrossRef]
- d’Alessio, V. Can We Reverse Antibiotic Resistance? 21 October 2019. Available online: https://horizon-magazine.eu/article/can-we-reverse-antibiotic-resistance.html (accessed on 1 December 2019).
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Iman, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Veras, H.N.H.; Rodrigues, F.F.G.; Corale, A.V.; Menezes, I.R.A.; Coutinho, H.D.M.; Boetelho, M.A.; Costa, J.G.M. Synergistic antibiotic activity of volatile compounds from the essential oil of Lippia sideroids and thymol. Fitoterapia 2012, 83, 508–512. [Google Scholar] [CrossRef] [Green Version]
- Sekar, D.; Kumar, V.; Muthukumar, H.; Gopinath, P.; Matheswaran, M. Electrospinning of Fe-doped ZnO nanoparticles incorporated polyvinyl alcohol nanofibers for its Antibacterial treatment and cytotoxic studies. Eur. Polym. J. 2019. [Google Scholar] [CrossRef]
- Huang, Y.; Song, J.; Yamg, C.; Long, Y.; Wu, L. Scalable manufacturing and applications of nanofibers. Mater. Today 2019, 28, 98–113. [Google Scholar] [CrossRef]
- Yao, C.-H.; Lee, C.-Y.; Huang, C.-H.; Chen, Y.-S.; Chen, K.-Y. Novel bilayer wound dresing based on electrospun gelatin/keratin nanofibrous mats for skin wound repair. Mater. Sci. Eng. C 2017, 79, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Harada, O. Preparation and characterization of collagen spun from liquid-crystallin collagen. In Proceedings of the 11th Asian International Conference of Leather Science and Technology, Xi’an, China, 17–19 October 2018; pp. 347–351. [Google Scholar]
- Tampan, A.; Gonzales-Martinez, C.; Chiralt, A. Carvacol encapsulation in starch or PCL based matrices by electrospinning. J. Food Eng. 2017, 214, 245–256. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.M.; Fahti, M. Encapsulation of orange oil using cross-linking electrospun gelatin nanofibers. Food Bioprocess Technol. 2018, 11, 427–434. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: London, UK, 2011; Available online: http://www.intechopen.com/books/biomaterials-applications-for-nanomedicine/collagen-vs-gelatine-based-biomaterials-and-their-biocompatibility-review-and-perspectives (accessed on 15 December 2019).
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buetlnes, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of electrospun poly(ɛ-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Moreno, P.J.; Guadix, P.J.; Guadix, E.M.; Jacobseu, C. Physical and oxidative stability of fish oil-in-water emulsion stabilized with fish protein hydrolysates. Food Chem. 2016, 203, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Mori, C.I.; Passos, N.A.D.; Oliviera, J.E.; Aloe, T.E.; Mori, F.A.; Molttoso, L.H.C.; Tonoli, G.H.D. Nanostructured polylactic acid/candeia essential oil mats obtained by electrospinning. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Goncalvesa, M.J.; Cruzb, M.T.; Cavaleiroa, C.; Lopesb, M.C.; Salgueiroa, L. Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp. Sylvestris. Ind. Crop. Prod. 2010, 32, 70–75. [Google Scholar] [CrossRef]
- Ksouri, S.; Djebir, S.; Bentorki, A.A.; Gouri, A.; Hadef, Y.; Benakhla, A. Antifungal activity of essential oils extract from Origanum floribundum Munby, Rosmarinus officinalis L. and Thymus ciliatus Desf. against Candida albicans isolated from bovine clinical mastitis. J. Mycol. Médicale 2017, 27, 245–249. [Google Scholar] [CrossRef]
- Bousmaha-Marroki, L.; Alik-Bekkara, F.; Tomi, F.; Casanova, J. Chemical composition and antibacterial activity of essential oil of Thymus ciliatus (Desf.)/Benth. ssp. eu-ciliatus Maire from Algeria. J. Essent. Oil Res. 2007, 19, 490–493. [Google Scholar] [CrossRef]


















| Characteristics, U.M. | Value ± Standard Deviation |
|---|---|
| Dry matter, % | 60.40 ± 0.42 |
| Ash a, % | 6.24 ± 0.27 |
| Total nitrogen a, % | 14.67 ± 0.66 |
| Protein a, % | 82.43 ± 2.66 |
| pH, pH units | 8.54 ± 0.10 |
| Aminic nitrogen b, % | 1.43 ± 0.06 |
| Electrical conductivity, μs/cm | 870 ± 0.1 |
| Element | P1 | P2 | P3 | |||
|---|---|---|---|---|---|---|
| Mass (%) | Atom (%) | Mass (%) | Atom (%) | Mass (%) | Atom (%) | |
| Carbon | 38.08 | 54.18 | 41.33 | 59.68 | 47.53 | 58.63 |
| Oxygen | 10.40 | 11.11 | 9.22 | 9.99 | 15.30 | 14.17 |
| Nitrogen | 19.38 | 23.66 | 15.75 | 19.50 | 21.85 | 23.11 |
| Chlorine | 4.24 | 2.04 | 4.34 | 2.12 | 2.09 | 0.87 |
| Calcium | 14.20 | 6.05 | 14.58 | 6.31 | 6.13 | 2.27 |
| Sodium | 1.78 | 1.32 | 1.63 | 1.23 | 0.73 | 0.47 |
| Expression of Inhibition Zone Diameters | |||||
|---|---|---|---|---|---|
| Samples | S. aureus ATCC 25923 | E. coli ATCC 25922 | P. aeruginosa ATCC 27853 | Samples | C. albicans ATCC 10231 |
| P1 | +/− − | +/− − | +/− − | P1 | +/− − |
| P2 | +/− | + | +/− | P2 | + |
| P3 | +/− | + | +/− | P3 | + |
| Gentamycin | + | + | + | Amphotericin | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Râpă, M.; Stanca, M.; Ditu, L.-M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials 2020, 13, 1618. https://doi.org/10.3390/ma13071618
Berechet MD, Gaidau C, Miletic A, Pilic B, Râpă M, Stanca M, Ditu L-M, Constantinescu R, Lazea-Stoyanova A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials. 2020; 13(7):1618. https://doi.org/10.3390/ma13071618
Chicago/Turabian StyleBerechet, Mariana Daniela, Carmen Gaidau, Aleksandra Miletic, Branka Pilic, Maria Râpă, Maria Stanca, Lia-Mara Ditu, Rodica Constantinescu, and Andrada Lazea-Stoyanova. 2020. "Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils" Materials 13, no. 7: 1618. https://doi.org/10.3390/ma13071618
APA StyleBerechet, M. D., Gaidau, C., Miletic, A., Pilic, B., Râpă, M., Stanca, M., Ditu, L. -M., Constantinescu, R., & Lazea-Stoyanova, A. (2020). Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials, 13(7), 1618. https://doi.org/10.3390/ma13071618