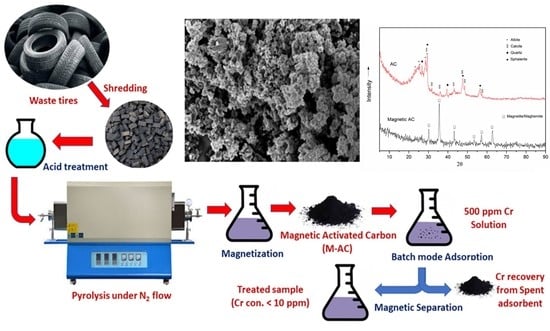
Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater
Abstract
:1. Introduction
2. Experimental Work
2.1. Chemicals and Reagents
2.2. Sample and Materials Collection
2.3. Preparation of Model Cr Solution
2.4. Preparation of the Adsorbent
2.4.1. Preparation of Activated Carbon
2.4.2. Magnetization of Activated Carbon
2.5. Characterization of Adsorbent
2.6. Batch Adsorption Experiments
2.7. Recovery of Cr from Adsorbent
2.8. Analysis of Cr in Water Sample
2.9. Calculations
3. Results and Discussion
3.1. Characterization of Adsorbent
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
3.1.3. FESEM Analysis
3.1.4. EDX Analysis
3.2. Adsorption Experiments and Effect of Process Parameters
3.3. Kinetic Study
3.4. Thermodynamic Studies
3.5. Adsorption Isotherms
3.6. Recovery of Cr(VI) and Regeneration of Adsorbent
3.7. Mechanism of Adsorption
3.8. Treatment of Tannery Wastewater
3.9. Comparison of Adsorption Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batool, A.; Saleh, T.A. Removal of toxic metals from wastewater in constructed wetlands as a green technology; catalyst role of substrates and chelators. Ecotoxicol. Environ. Saf. 2020, 189, 109924. [Google Scholar] [CrossRef] [PubMed]
- Mella, B.; Puchana-Rosero, M.; Costa, D.; Gutterres, M. Utilization of tannery solid waste as an alternative biosorbent for acid dyes in wastewater treatment. J. Mol. Liq. 2017, 242, 137–145. [Google Scholar] [CrossRef]
- Kokkinos, E.; Proskynitopoulou, V.; Zouboulis, A. Chromium and energy recovery from tannery wastewater treatment waste: Investigation of major mechanisms in the framework of circular economy. J. Environ. Chem. Eng. 2019, 7, 103307. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.; Jaafar, J.; Ismail, A. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Xiao, K.; Han, G.; Li, J.; Dan, Z.; Xu, F.; Jiang, L.; Duan, N. Evaluation of polyacrylic anion exchange resins on the removal of Cr (VI) from aqueous solutions. RSC Adv. 2016, 6, 5233–5239. [Google Scholar] [CrossRef]
- Wójcik, G. Sorption and reduction of chromium ions by the chelating ion exchanger Diaion CR20. Physicochem. Probl. Miner. Process. 2019, 55, 1382–1393. [Google Scholar]
- Park, D.; Yun, Y.-S.; Park, J.M. Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere 2005, 60, 1356–1364. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman, C.U., Jr.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Adefa, T.; Tefera, M. Heavy Metal Accumulation and Health Risk Assessment in Moringa Oleifera from Awi Zone, Ethiopia. Chem. Afr. 2020, 3, 1073–1079. [Google Scholar] [CrossRef]
- Geng, B.; Jin, Z.; Li, T.; Qi, X. Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere 2009, 75, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Zarrinmehr, M.J.; Kousha, M.; Hashtjin, A.M.; Saratale, G.D.; Maiti, A.; Vithanage, M.; Bhatnagar, A. Hexavalent chromium removal from water by microalgal-based materials: Adsorption, desorption and recovery studies. Bioresour. Technol. 2019, 293, 122064. [Google Scholar] [CrossRef] [PubMed]
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Singhal, A.; Kumari, P. Exploring carbonaceous nanomaterials for arsenic and chromium removal from wastewater. J. Water Process Eng. 2020, 36, 101276. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Liao, N.; Kim, J.H.; Kim, H.J.; Park, C.H.; Kim, C.S. In-situ deposition of silver− iron oxide nanoparticles on the surface of fly ash for water purification. J. Colloid Interface Sci. 2015, 453, 159–168. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined graphene/MgAl-layered double hydroxides for enhanced Cr (VI) removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.; Mutalib, M.; Ismail, A.; Salleh, W.; Jaafar, J.; Yusof, N.; Ludin, N. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Briton, B.G.H.; Duclaux, L.; Richardson, Y.; Yao, K.B.; Reinert, L.; Soneda, Y. Effectiveness of the dispersion of iron nanoparticles within micropores and mesopores of activated carbon for Rhodamine B removal in wastewater by the heterogeneous Fenton process. Appl. Water Sci. 2019, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Feizi, F.; Reguyal, F.; Antoniou, N.; Zabaniotou, A.; Sarmah, A.K. Environmental remediation in circular economy: End of life tyre magnetic pyrochars for adsorptive removal of pharmaceuticals from aqueous solution. Sci. Total Environ. 2020, 739, 139855. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef] [PubMed]
- Adio, S.O.; Asif, M.; Mohammed, A.-R.I.; Baig, N.; Al-Arfaj, A.A.; Saleh, T.A. Poly (amidoxime) modified magnetic activated carbon for chromium and thallium adsorption: Statistical analysis and regeneration. Process Saf. Environ. Prot. 2019, 121, 254–262. [Google Scholar] [CrossRef]
- Raji, C.; Anirudhan, T. Batch Cr (VI) removal by polyacrylamide-grafted sawdust: Kinetics and thermodynamics. Water Res. 1998, 32, 3772–3780. [Google Scholar] [CrossRef]
- Lace, A.; Ryan, D.; Bowkett, M.; Cleary, J. Chromium Monitoring in Water by Colorimetry Using Optimised 1,5-Diphenylcarbazide Method. Int. J. Environ. Res. Public Health 2019, 16, 1803. [Google Scholar] [CrossRef] [Green Version]
- Avila, M.; Burks, T.; Akhtar, F.; Göthelid, M.; Lansåker, P.C.; Toprak, M.S.; Muhammed, M.; Uheida, A. Surface functionalized nanofibers for the removal of chromium (VI) from aqueous solutions. Chem. Eng. J. 2014, 245, 201–209. [Google Scholar] [CrossRef]
- Khan, M.; Yilmaz, E.; Sevinc, B.; Sahmetlioglu, E.; Shah, J.; Jan, M.R.; Soylak, M. Preparation and characterization of magnetic allylamine modified graphene oxide-poly (vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta 2016, 146, 130–137. [Google Scholar] [CrossRef]
- Kandile, N.G.; Nasr, A.S. New hydrogels based on modified chitosan as metal biosorbent agents. Int. J. Biol. Macromol. 2014, 64, 328–333. [Google Scholar] [CrossRef]
- Yousaf, I.; Rahman, U.; Mansoor, K. Solid phase extraction of Pb (II) and Cd (II) using reduced graphene oxide-polychloroprene impregnated with magnetic nanoparticle (MNPs-RGO-PCP). Desalin. Water Treat. 2018, 114, 232–241. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.-U.-H.A.; Bilal, S.; Rahman, G. Basic Blue dye adsorption from water using Polyaniline/Magnetite (Fe3O4) composites: Kinetic and thermodynamic aspects. Materials 2019, 12, 1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Das, M.R. Removal of a cationic dye from aqueous solution using graphene oxide nanosheets: Investigation of adsorption parameters. J. Chem. Eng. Data 2013, 58, 151–158. [Google Scholar] [CrossRef]
- Karmacharya, M.S.; Gupta, V.K.; Jha, V.K. Preparation of activated carbon from waste tire rubber for the active removal of Cr (VI) and Mn (II) ions from aqueous solution. Trans. Indian Ceram. Soc. 2016, 75, 234–241. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Pezoti, O.; Bedin, K.C.; Silva, T.L.; Paesano Junior, A.; Asefa, T.; Almeida, V.C. Magnetic Activated Carbon Derived from Biomass Waste by Concurrent Synthesis: Efficient Adsorbent for Toxic Dyes. ACS Sustain. Chem. Eng. 2016, 4, 1058–1068. [Google Scholar] [CrossRef]
- Nunes, M.R.; Perez, G.M.; Loguercio, L.F.; Alves, E.W.; Carreño, N.L.V.; Martins, J.L.; Garcia, I.T.S. Active carbon preparation from treads of tire waste for dye removal in waste water. J. Braz. Chem. Soc. 2011, 22, 2027–2035. [Google Scholar] [CrossRef] [Green Version]
- Jha, V.K.; Subedi, K. Preparation of activated charcoal adsorbent from waste tire. J. Nepal Chem. Soc. 2011, 27, 19–25. [Google Scholar] [CrossRef]
- Dermentzis, K.; Christoforidis, A.; Valsamidou, E.; Lazaridou, A.; Kokkinos, N. Removal of hexavalent chromium from electroplating wastewater by electrocoagulation with iron electrodes. Glob. Nest J. 2011, 13, 412–418. [Google Scholar]
- Lu, X.; Li, M.; Deng, H.; Lin, P.; Matsumoto, M.R.; Liu, X. Application of electrochemical depassivation in PRB systems to recovery Fe 0 reactivity. Front. Environ. Sci. Eng. 2016, 10, 4. [Google Scholar] [CrossRef]
- Wang, J.; Pan, K.; He, Q.; Cao, B. Polyacrylonitrile/polypyrrole core/shell nanofiber mat for the removal of hexavalent chromium from aqueous solution. J. Hazard. Mater. 2013, 244, 121–129. [Google Scholar] [CrossRef]
- Tran, T.K.; Leu, H.J.; Vu, T.Q.; Nguyen, M.T.; Pham, T.A.; Kiefer, R. Hydrogen production from the tannery wastewater treatment by using agriculture supports membrane/adsorbents electrochemical system. Int. J. Hydrog. Energy 2020, 45, 3699–3711. [Google Scholar] [CrossRef]
- Song, Z.; Williams, C.; Edyvean, R. Treatment of tannery wastewater by chemical coagulation. Desalination 2004, 164, 249–259. [Google Scholar] [CrossRef]
- Chun, L.; Hongzhang, C.; Zuohu, L. Adsorptive removal of Cr (VI) by Fe-modified steam exploded wheat straw. Process Biochem. 2004, 39, 541–545. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Removal of chromium (VI) from aqueous solutions by polymer inclusion membranes. Water Res. 2002, 36, 4870–4876. [Google Scholar] [CrossRef]
- Hussain, S.; Gul, S.; Khan, S.; ur Rehman, H. Retention studies of chromium (VI) from aqueous solution on the surface of a novel carbonaceous material. Arab. J. Geosci. 2013, 6, 4547–4556. [Google Scholar] [CrossRef]
- Demarchi, C.A.; Michel, B.S.; Nedelko, N.; Ślawska-Waniewska, A.; Dłużewski, P.; Kaleta, A.; Minikayev, R.; Strachowski, T.; Lipińska, L.; Dal Magro, J.; et al. Preparation, characterization, and application of magnetic activated carbon from termite feces for the adsorption of Cr(VI) from aqueous solutions. Powder Technol. 2019, 354, 432–441. [Google Scholar] [CrossRef]
- Garg, V.; Gupta, R.; Kumar, R.; Gupta, R. Adsorption of chromium from aqueous solution on treated sawdust. Bioresour. Technol. 2004, 92, 79–81. [Google Scholar] [CrossRef]
- Attia, A.; Khedr, S.; Elkholy, S. Adsorption of chromium ion (VI) by acid activated carbon. Braz. J. Chem. Eng. 2010, 27, 183–193. [Google Scholar] [CrossRef]
- Mondal, N.K.; Chakraborty, S. Adsorption of Cr(VI) from aqueous solution on graphene oxide (GO) prepared from graphite: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Ullah, R.; Ahmad, W.; Ahmad, I.; Khan, M.; Iqbal Khattak, M.; Hussain, F. Adsorption and recovery of hexavalent chromium from tannery wastewater over magnetic max phase composite. Sep. Sci. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, F.; Peng, Z. Adsorption mechanism of Cr (VI) onto GO/PAMAMs composites. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shakoor, S.; Nasar, A. Adsorptive decontamination of synthetic wastewater containing crystal violet dye by employing Terminalia arjuna sawdust waste. Groundw. Sustain. Dev. 2018, 7, 30–38. [Google Scholar] [CrossRef]
- Salem, M.A. The role of polyaniline salts in the removal of direct blue 78 from aqueous solution: A kinetic study. React. Funct. Polym. 2010, 70, 707–714. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M. Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; He, M.; Chen, B. Nanometer-sized materials for solid-phase extraction of trace elements. Anal. Bioanal. Chem. 2015, 407, 2685–2710. [Google Scholar] [CrossRef]
- Vuong Hoan, N.T.; Anh Thu, N.T.; Duc, H.V.; Cuong, N.D.; Quang Khieu, D.; Vo, V. Fe3O4/reduced graphene oxide nanocomposite: Synthesis and its application for toxic metal ion removal. J. Chem. 2016, 2016, 2418172. [Google Scholar] [CrossRef] [Green Version]
- Amuda, O.; Ibrahim, A. Industrial wastewater treatment using natural material as adsorbent. Afr. J. Biotechnol. 2006, 5, 1483–1487. [Google Scholar]
- Jain, M.; Yadav, M.; Kohout, T.; Lahtinen, M.; Garg, V.K.; Sillanpää, M. Development of iron oxide/activated carbon nanoparticle composite for the removal of Cr(VI), Cu(II) and Cd(II) ions from aqueous solution. Water Resour. Ind. 2018, 20, 54–74. [Google Scholar] [CrossRef]
- Valentín-Reyes, J.; García-Reyes, R.B.; García-González, A.; Soto-Regalado, E.; Cerino-Córdova, F. Adsorption mechanisms of hexavalent chromium from aqueous solutions on modified activated carbons. J. Environ. Manag. 2019, 236, 815–822. [Google Scholar] [CrossRef]
- Kurniawan, T.; Babel, S. A research study on Cr (VI) removal from contaminated wastewater using low-cost adsorbents and commercial activated carbon. In Proceedings of the Second Int. Conf. on Energy Technology towards a Clean Environment (RCETE), Phuket, Thailand, 12–14 February 2003; pp. 1110–1117. [Google Scholar]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer′Eb, M. Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Kumar, S.; Meikap, B. Removal of chromium (VI) from waste water by using adsorbent prepared from green coconut shell. Desalin. Water Treat. 2014, 52, 3122–3132. [Google Scholar] [CrossRef]
- Fahim, N.; Barsoum, B.; Eid, A.; Khalil, M. Removal of chromium (III) from tannery wastewater using activated carbon from sugar industrial waste. J. Hazard. Mater. 2006, 136, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Renu, M.A.; Singh, K.; Upadhyaya, S.; Dohare, R. Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater. Today Proc. 2017, 4, 10534–10538. [Google Scholar] [CrossRef]
- Dubey, S.P.; Gopal, K. Adsorption of chromium (VI) on low cost adsorbents derived from agricultural waste material: A comparative study. J. Hazard. Mater. 2007, 145, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Andrews, J.L.; Steed, J.W.; D’Anna, F. Carbohydrate-supramolecular gels: Adsorbents for chromium (VI) removal from wastewater. J. Colloid Interface Sci. 2019, 548, 184–196. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Bi, C.; Zeng, C.; Ma, W.; Yan, L.; Li, K.; Wei, K. Camellia oleifera seed shell carbon as an efficient renewable bio-adsorbent for the adsorption removal of hexavalent chromium and methylene blue from aqueous solution. J. Mol. Liq. 2018, 249, 629–636. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, B.; Li, M.; Li, C.; Zheng, S. Carboxyl-rich carbon nanocomposite based on natural diatomite as adsorbent for efficient removal of Cr (VI). J. Mater. Res. Technol. 2020, 9, 948–959. [Google Scholar] [CrossRef]








| Element | AC | Magnetic AC | ||
|---|---|---|---|---|
| Wt% | At% | Wt% | At% | |
| C | 75.98 | 85.53 | 45.81 | 66.05 |
| O | 08.63 | 07.30 | 21.43 | 23.20 |
| Na | 03.08 | 01.81 | - | - |
| Al | 01.41 | 00.71 | - | - |
| Si | 04.94 | 02.38 | 01.25 | 00.77 |
| S | 03.08 | 01.30 | 00.89 | 00.48 |
| K | 01.44 | 00.50 | - | - |
| Ca | 01.44 | 00.48 | - | - |
| Fe | - | - | 30.62 | 09.50 |
| Experimental | Pseudo First Order | Pseudo Second Order | Intra-Particle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (mg·g−1) | K1 (min−1) | qe (mg·g−1) | R2 | K2 (min−1) | qe (mg·g−1) | R2 | Ki (g/mg.min) | C | R2 |
| 49.314 | 0.064 | 30.63 | 0.97 | 0.0023 | 58.82 | 0.99 | 0.024 | 0.008 | 0.97 |
| Temperature (K) | ΔGᵒ (KJmol−1) | ΔHᵒ (KJmol−1) | ΔSᵒ (KJmol−1k−1) |
|---|---|---|---|
| 298 | −21.58 | 26.069 | 0.162 |
| 308 | −24.54 | ||
| 318 | −25.43 | ||
| 328 | −27.68 | ||
| 338 | −28.56 | ||
| 348 | −29.92 |
| Isotherm Model | R2 | Kf | 1/n | Kb | qm mg·g−1 | RL |
|---|---|---|---|---|---|---|
| Langmuir Isotherm | 0.996 | - | - | 0.303 | 142.85 | - |
| Freundlich Isotherm | 0.828 | 0.242 | 0.168 | - | - | 0.352 |
| Leaching Solution | Cr (VI) Recovery (%) |
|---|---|
| 1 M NaOH | 45 ± 2 |
| 2 M NaOH | 56 ± 1 |
| 1 M NH4OH | 30 ± 2 |
| 2 M NH4OH | 35 ± 2 |
| Parametre | Values |
|---|---|
| pH | 3.17 |
| COD | 1130 mg/L |
| Suspended solids | 960 mg/L |
| Concentration of Cr | 1640 mg/L |
| Removal of Cr through adsorption | 97% |
| Adsorbent Materials | Experimental Conditions | Adsorption Capacity | Reference |
|---|---|---|---|
| Acid activated Carbon derived from olive stones | pH 1.5, Cr conc. in water 4–50 mg/L, Adsorbent dose 0.3 g | 71 mg/g | [47] |
| Coconut shell charcoal (CSC) and commercial activated carbon (CAC) | Cr conc. 5,10, 20, and 25 mg/L | CAC at 4.7 mg/g & CSC at 5 mg/L | [59] |
| Wool, sawdust, pine needles, almond shells, cactus leaves, and charcoal | Cr conc. 20, 100, 200, 300, 400, 500 and 1000 mg l−1, Adsorbent conc. 16 g l−1 at 30 °C | 81% out of 100 ppm Cr(VI) | [60] |
| Green coconut shell | Cr conc. 10–100 mg/g, T range 10–80 °C | 22.9 mg/g (90% for 10 mg/L) | [61] |
| Activated carbon (C1, C2, C3) from industrial sugar waste | pH 5–6, T 28 °C, Cr conc. 0.15 and 0.7 mg/L | C1 98.86, C2 98.6 and C3 93% | [62] |
| Activated carbon (1), calcinated egg shells (2), wheat bran (3), modified wheat bran (4) | Cr conc. 10 mg/L, rpm-180, T 35 °C | 98.75% (1), 64% (2), 75.89% (3), 96.96% (4) | [63] |
| Silver impregnated groundnut husk (1), activated carbon from groundnut husk (2) | pH 1–3, Cr conc. 0.5 g/100 mL | 11.4 mg/g (1), 7.0104 mg/g (2) | [64] |
| Inexpensive carbohydrates derived supramolecular gels | pH 7.4, 250 mg or 20 mg of gel at 5 wt% in 10 or 20 mL Cr solutions | 598 mg/g (97% in 24 h) | [65] |
| Porous activated carbon from Camellia oleifera seed | pH 2–8, Cr conc. 30 mg L−1, T 25 °C | 307.3 mg/g | [66] |
| Carbon nanocomposite from natural diatomite | pH 1, Cr conc. 50–300 mg/L, T 298.15 K | 142.9 mg/g | [67] |
| Magnetic activated carbon (M-AC) from tires waste | pH 2, 35 °C, 40 min, 100 mg adsorbent | 49.3 mg/g | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Qaiser, S.; Ullah, R.; Mohamed Jan, B.; Karakassides, M.A.; Salmas, C.E.; Kenanakis, G.; Ikram, R. Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater. Materials 2021, 14, 34. https://doi.org/10.3390/ma14010034
Ahmad W, Qaiser S, Ullah R, Mohamed Jan B, Karakassides MA, Salmas CE, Kenanakis G, Ikram R. Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater. Materials. 2021; 14(1):34. https://doi.org/10.3390/ma14010034
Chicago/Turabian StyleAhmad, Waqas, Shanif Qaiser, Rahman Ullah, Badrul Mohamed Jan, Michael A. Karakassides, Constantinos E. Salmas, George Kenanakis, and Rabia Ikram. 2021. "Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater" Materials 14, no. 1: 34. https://doi.org/10.3390/ma14010034
APA StyleAhmad, W., Qaiser, S., Ullah, R., Mohamed Jan, B., Karakassides, M. A., Salmas, C. E., Kenanakis, G., & Ikram, R. (2021). Utilization of Tires Waste-Derived Magnetic-Activated Carbon for the Removal of Hexavalent Chromium from Wastewater. Materials, 14(1), 34. https://doi.org/10.3390/ma14010034