Recent Developments in Lactone Monomers and Polymer Synthesis and Application
Abstract
:1. Introduction
2. Properties of Lactones
3. Methods for the Synthesis of Lactones
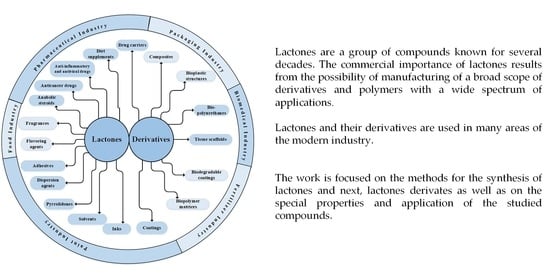
4. Products Produced from Lactones and Their Applications
5. Methods for the Synthesis of Lactones Derivatives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fortman, D.J.; Brutman, J.P.; De Hoe, G.X.; Snyder, R.L.; Dichtel, W.R.; Hillmyer, M.A. Approaches to Sustainable and Continually Recyclable Cross-Linked Polymers. ACS Sustain. Chem. Eng. 2018, 6, 11145–11159. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Cao, H.; Robertson, M.L. Hydrolysis and Solvolysis as Benign Routes for the End-of-Life Management of Thermoset Polymer Waste. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 183–201. [Google Scholar] [CrossRef] [Green Version]
- Pelouze, J. Memoir on lactic acid. Comptes Rendus 1844, 19, 1219–1227. [Google Scholar]
- Fittig, R.; Liepmann, H.I. Ueber das Fluoranthen, einen neuen Kohlenwasserstoff im Steinkohlentheer. Eur. J. Org. Chem. 1880, 200, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G. Poly(ε-caprolactone)-Clay Nanocomposites: Structure and Mechanical Properties. Macromolecules 2006, 39, 747–754. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69. [Google Scholar] [CrossRef]
- Löfgren, A.; Albertsson, A.-C.; Dubois, P.; Jerôme, R. Recent Advances in Ring-Opening Polymerization of Lactones and Related Compounds. J. Macromol. Sci. Part C 1995, 35, 379–418. [Google Scholar] [CrossRef]
- LeComte, P.; Jérôme, C. Recent Developments in Ring-Opening Polymerization of Lactones. Adv. Polym. Sci. 2011, 245, 173–217. [Google Scholar] [CrossRef]
- Janecki, T. Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity; John Wiley & Sons: Weinheim, Germany, 2014. [Google Scholar]
- Libiszewska, K. Lactones as biologically active compounds. Biotechnol. Food Sci. 2011, 75, 45–53. [Google Scholar]
- Novomer. Available online: https://www.novomer.com/product (accessed on 21 April 2021).
- BASF. Available online: https://chemicals.basf.com/global/en/Intermediates/Product_groups/Lactones_Lactams.html (accessed on 21 April 2021).
- Gantrade. Available online: https://www.gantrade.com/blog/caprolactone-monomer-a-gateway-building-block-for-advanced-performance-intermediates (accessed on 26 March 2021).
- WHO. Gamma-butyrolactone (GBL) Critical Review Report Agenda item 4.3. Expert Committee on Drug Dependence Thirty-sixth Meeting; World Health Assembly; Geneva, Switzerland, 2014. [Google Scholar]
- Getzler, Y.D.Y.L.; Kundnani, V.; Lobkovsky, E.B.; Coates, G.W. Catalytic Carbonylation of β-Lactones to Succinic Anhydrides. J. Am. Chem. Soc. 2004, 126, 6842–6843. [Google Scholar] [CrossRef]
- Fragrantica. Available online: https://www.fragrantica.com/news/Peaches-Coconuts-and-Cream-Lactones-in-Fragrance-11529.html (accessed on 21 April 2021).
- Li, Y.; Yin, X.; Dai, M. Catalytic macrolactonizations for natural product synthesis. Natural Prod. Rep. 2017, 34, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Sitko, M.; Szelwicka, A.; Szmatoła, M.; Skwarek, A.; Tadasiewicz, D.; Schimmelpfennig, L.; Dziuba, K.; Morawiec-Witczak, M.; Iłowska, A. Chrobok, Method of the synthesis of ε-caprolactone in the chemo-enzymatic oxidation of cyclohexanone in emulsion. Chem. Eng. Mater. Res. Inf. Cent. 2019, 98, 1587–1593. [Google Scholar] [CrossRef]
- Paryzek, Z.; Skiera, I. Synthesis and cleavage of lactones and thiolactones. Applications inorganic synthesis. A review. Organic Preparations and Procedures International. N. J. Org. Synth. 2007, 39, 203–296. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, G.; Lin, L. Preparation of δ-Valerolactone and Its Spectral Analysis. U.S. Patent 2011/0237806A1, 31 October 2017. [Google Scholar]
- Szelwicka, A.; Kolanowska, A.; Latos, P.; Jurczyk, S.; Boncel, S.; Chrobok, A. Carbon nanotube/PTFE as a hybrid platform for lipase B from Candida antarctica in transformation of α-angelica lactone into alkyl levulinates. Catal. Sci. Technol. 2020, 10, 3255–3264. [Google Scholar] [CrossRef]
- Allen, S.D.; Valente, R.R.; Lee, H.; Cherian, A.E.; Bunning, D.L.; Clinton, N.A.; Fruchey, O.S.; Dombek, B.D. Process for Beta-Lactone Production. U.S. Patent 2016/0102040A1, 16 February 2016. [Google Scholar]
- Schwarz, W.; Schossig, J.; Rossbacher, R.; Pinkos, R.; Höke, H. Butyrolactone. Ullmann’s Encycl. Ind. Chem. 2021. [Google Scholar] [CrossRef]
- Kanetaka, J.; Asano, T.; Masumune, S. New Process for Production of Tetrahydrofuran. Ind. Eng. Chem. 1970, 62, 24–32. [Google Scholar] [CrossRef]
- Tuck, M.W.M.; Wood, M.A.; Rathmell, C.; Eastland, P.H.E. Butane to butanediol: The emergence of a new process route. In AIChE Spring International Meeting; AIChE the Global Home of Chemical Engineers; New York, NY, USA, 1994. [Google Scholar]
- Young, F. Production of Beta-Hydroxy Carboxylic Acid Lactones From Ketene and Aldehyde with Clay Catalyst. U.S. Patent 2,580,714A, 1 January 1952. [Google Scholar]
- Renz, M.; Meunier, B. 100 Years of Baeyer-Villiger Oxidations. Eur. J. Org. Chem. 1999, 4, 737–750. [Google Scholar] [CrossRef]
- Brink, G.J.; Arends, I.W.C.E.; Sheldon, R.A. The Baeyer-Villiger Reaction: New Developments toward Greener Pocedures. Chem. Rev. 2004, 104, 4105–4123. [Google Scholar] [CrossRef]
- Krow, G. The Baeyer-Villiger Oxidation of Ketones and Aldehydes. Org. React. 1993, 43, 251–798. [Google Scholar]
- Philips, B.; Tinsiey, S.W.; Starcher, P.S. Epsilon-Caprolactones and Process for Preparing the same. U.S. Patent 3,064,008A, 19 September 1961. [Google Scholar]
- Kliff, V. Process for the Production of Epsilon-Caprolactones and Carboxylic Acids. U.S. Patent 3,025,306, 13 March 1962. [Google Scholar]
- Sitko, M.; Szelwicka, A.; Wojewódka, A.; Skwarek, A.; Tadasiewicz, D.; Schimmelpfennig, L.; Dziuba, K.; Morawiec-Witczak, M.; Chrobok, A. Perdecanoic acid as a safe and stable medium-chain peracid for Baeyer–Villiger oxidation of cyclic ketones to lactones. RSC Adv. 2019, 9, 30012–30018. [Google Scholar] [CrossRef] [Green Version]
- Baj, S.; Chrobok, A. Method of Obtatining Esters and Lactones in the Medium of Ionic Liquids. PL Patent 206,383B1, 2 February 2009. [Google Scholar]
- Page, Eilerman. Process for the Preparation of Gamma and Delta Lactones. WO Patent 89/12104, 14 December 1989.
- Spaninger, E.; Bren, U. Carcinogenesis of β-Propiolactone: A Computational Study. Chem. Res. Toxicol. 2020, 33, 769–781. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Arul, M.R.; Sardashti, N.; Stedman, T.; James, R.; Rudraiah, S.; Kumbar, S.G. Biodegradable Polymeric Injectable Implants for Long-Term Delivery of Contraceptive Drugs. J. Appl. Polym. Sci. 2018, 135, 46068. [Google Scholar] [CrossRef]
- Caballero-George, C.; Marin, E.; Briceño, M.I. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hege, C.S.; Schiller, S.M. Non-toxic catalysts for ring-opening polymerizations of biodegradable polymers at room temperature for biohybrid materials. Green Chem. 2014, 16, 1410–1416. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Zięba, M.; Klim, M.; Kurcok, P. Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides: The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism. Polymers 2019, 11, 1221. [Google Scholar] [CrossRef] [Green Version]
- Monsalve1, M.; Contreras, J.M.; Laredo, E.; López-Carrasquero, F. Ring-opening copolymerization of (R,S)-β-butyrolactone and ε-caprolactone using sodium hydride as initiator. Express Polym. Lett. 2010, 4, 431–441. [Google Scholar] [CrossRef]
- Kiriratnikom, J.; Robert, C.; Guérineau, V.; Venditto, V.; Thomas, C.M. Stereoselective Ring-Opening (Co)polymerization of β-Butyrolactone and ε-Decalactone Using an Yttrium Bis(phenolate) Catalytic System. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Fagerland, J.; Finne-Wistranda, A.; Pappalardo, D. Modulating the thermal properties of poly(hydroxybutyrate) by the copolymerization of rac-β-butyrolactone with lactide. N. J. Chem. 2016, 40, 7671–7679. [Google Scholar] [CrossRef] [Green Version]
- Grobelny, Z.; Jurek-Suliga, J.; Golba, S. New way of anionic ring-opening copolymerization of β-butyrolactone and ε-caprolactone: Determination of the reaction course. J. Polym. Res. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Jaffredo, C.G.; Carpentier, J.-F.; Guillaume, S.M. Poly(hydroxyalkanoate) Block or Random Copolymers of β-Butyrolactone and Benzyl β-Malolactone: A Matter of Catalytic Tuning. Macromolecues 2013, 46, 6765–6776. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Zhao, Z.; Zhao, N.; Liu, F.; Li, Z. Preparation of Amphiphilic Poly(ethylene glycol)-b-poly(γ-butyrolactone) Diblock Copolymer via Ring Opening Polymerization Catalyzed by a Cyclic Trimeric Phosphazene Base or Alkali Alkoxide. Biomacromolecules 2018, 20, 141–148. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, J.; Zhao, N.; Liu, F.; Li, Z. Preparation of biorenewable poly(γ-butyrolactone)-b-poly(l-lactide) diblock copolyesters via one-pot sequential metal-free ring-opening polymerization. Polym. Chem. 2018, 9, 2936–2941. [Google Scholar] [CrossRef]
- Urbánek, T.; Trousil, J.; Rak, D.; Gunár, K.; Konefał, R.; Šlouf, M.; Sedlák, M.; Šebestová Janoušková, O.; Hrubý, M. γ-Butyrolactone Copolymerization with the Well-Documented Polymer Drug Carrier Poly (ethylene oxide)-block-poly(ε-caprolactone) to Fine-Tune Its Biorelevant Properties. Macromol. Biosci. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, C.; Manabe, S.; Nakayama, S.; Nakayama, Y.; Shiono, T. Impregnation of poly(L-lactide-ran-δ-valerolactone) with essential bark oil using supercritical carbon dioxide. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Deng, X.; Yang, H. Biodegradable poly(ε-caprolactone)-poly (ethylene glycol) block copolymers: Characterization and their use as drug carriers for a controlled delivery system. Biomaterials 2003, 24, 3563–3570. [Google Scholar] [CrossRef]
- Wang, H.; Synatschke, C.V.; Raup, A.; Jerome, V.; Freitag, R.; Agarwal, S. Oligomeric dual functional antibacterial polycaprolactone. Polym. Chem. 2014, 5, 2453–2460. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Someya, Y.; Tsukao, R. Nanocomposites based on poly(ε-caprolactone) and the montmorillonite treated with dibutylamine-terminated ε-caprolactone oligomer. J. Appl. Polym. Sci. 2007, 104, 3112–3119. [Google Scholar] [CrossRef]
- Saretia, S.; Machatschek, R.; Schulz, B.; Lendlein, A. Reversible 2D networks of oligo(ε-caprolactone) at the air–water interface. Biomed. Mater. 2019, 14, 034103. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rexach, E.; Iturri, J.; Fernandez, J.; Meaurio, E.; Toca-Herrera, J.L.; Sarasua, J.R. Novel biodegradable and non-fouling systems for controlled-release based on poly(ε-caprolactone)/Quercetin blends and biomimetic bacterial S-layer coatings. RSC Adv. 2019, 9, 24154–24163. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Wu, S.C.; Chen, H.; Tsai, L.L.; Tzeng, J.J.; Lin, C.H.; Lin, Y.M. Synthesis and Characterization of Polycaprolactone-Based Polyurethanes for the Fabrication of Elastic Guided Bone Regeneration Membrane. BioMed Res. Int. 2018. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Auzmendi, O.; Amestoy, H.; Diez-Torre, A.; Sarasua, J.R. Mechanical properties and fatigue analysis on poly (ε-caprolactone)-polydopamine-coated nanofibers and poly (ε-caprolactone)-carbon nanotube composite scaffolds. Eur. Polym. J. 2017, 94, 208–221. [Google Scholar] [CrossRef]
- Daicel. Available online: https://www.daicel.com/yuuki/en/product/index.php?act=list_view (accessed on 26 March 2021).
- Ingevity. Available online: https://www.ingevity.com/featured-products/capa-polyols/?gclid=Cj0KCQjw9_mDBhCGARIsAN3PaFPn0Rsxn-H2SH8pdaPm26lJauGncLZ3j-oI_68DxVqkIIIoeKBlZTEaAkjMEALw_wcB (accessed on 21 April 2021).
- Fernandez, J.; Etxeberria, A.; Sarasua, J.R. Synthesis and properties of ω-pentadecalactone-co-δ-hexalactone copolymers: A biodegradable thermoplastic elastomer as an alternative to poly(ε-caprolactone). RSC Adv. 2016, 6, 3137–3149. [Google Scholar] [CrossRef]
- Bansal, K.K.; Kakde, D.; Purdie, L.; Irvine, D.J.; Howdle, S.M.; Mantovani, G.; Alexander, C. New Biomaterials from Renewable Resources-Amphiphilic Block Copolymers from δ-Decalactone. Polym. Chem. 2015, 6, 7196–7210. [Google Scholar] [CrossRef] [Green Version]
- Schneiderman, D.K.; Hill, E.M.; Martello, M.T.; Hillmyer, M.A. Poly (lactide)-block-poly (ε-caprolactone-co-ε-decalactone)-block-poly (lactide) copolymer elastomers. Polym. Chem. 2015, 6, 3641–3651. [Google Scholar] [CrossRef]
- Kakde, D.; Taresco, V.; Bansal, K.K.; Magennis, E.P.; Howdle, S.M.; Mantovani, G.; Irvine, D.J.; Alexander, C. Amphiphilic block copolymers from a renewable ε-decalactone monomer: Prediction and characterization of micellar core effects on drug encapsulation and release. J. Mater. Chem. B 2016, 4, 7119–7129. [Google Scholar] [CrossRef]
- Ames, W.A. Copolymerization of Heterocyclic Monomers by Ring Scission. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 1958. [Google Scholar]
- Gresham, T.L.; Jansen, J.E.; Shaver, F.W. β-Propiolactone. I. Polymerization Reactions. J. Am. Chem. Soc. 1948, 70, 998–999. [Google Scholar] [CrossRef]
- Marans, N.S. Preparation of Poly-Beta-Propiolactone. U.S. Patent 3,111,469A, 19 November 1963. [Google Scholar]
- Tada, K.; Numata, Y.; Saegusa, T.; Furukawa, J. Copolymerization of y-Butyrolactone and β-Propiolactone. Macromol. Chem. Phys. 1964, 77, 220–228. [Google Scholar] [CrossRef]
- Nakayama, Y.; Aihara, K.; Cai, Z.; Shiono, T.; Tsutsumi, C. Synthesis and Biodegradation of Poly (L-lactide-co-propiolactone. Int. J. Mol. Sci. 2017, 18, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kricheldorf, H.R.; Mang, T.; Jonte, M. Copolymerization of glycolide with β-propiolactone, γ-butyrolactone or δ-valerolactone. Makromol. Chem. 1985, 186, 955–976. [Google Scholar] [CrossRef]
- Katayama, S.; Horikawa, S.; Horikawa, H.; Toshiria, O. Radical Copolymerizations of β-Propiolactone with Acrylonitrile and with Styrene. J. Polym. Sci. 1971, 1, 2915–2932. [Google Scholar] [CrossRef]
- Saegusa, T.; Ikeda, H.; Fujii, H. Alternating Copolymerization of 2-Oxazoline with β-Propiolactone. Macromolecues 1972, 5, 354–358. [Google Scholar] [CrossRef]
- Cerrai, P.; Tricoli, M.; Andruzzi, F. Synthesis and characterization of polymers from β-propiolactone and poly (ethylene glycol)s. Polymer 1987, 28, 831–836. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Tabernero, V.; Cuenca, T.; Mosquera, M.E.G.; Cano, J.; Milione, S. Biodegradable PHB from rac-β-Butyrolactone: Highly Controlled ROP Mediated by a Pentacoordinated Aluminum Complex. Organometallics 2018, 37, 837–840. [Google Scholar] [CrossRef]
- Couffin, A.; Martín-Vaca, B.; Bourissou, D.; Navarro, C. Selective O-acyl ring-opening of β-butyrolactone catalyzed by trifluoromethane sulfonic acid: Application to the preparation of well-defined block copolymers. Polym. Chem. 2014, 5, 161–168. [Google Scholar] [CrossRef]
- Lee, C.W.; Urakawa, R.; Kimura, Y. Copolymerization of γ-valerolactone and β-butyrolactone. Eur. Polym. J. 1998, 34, 117–122. [Google Scholar] [CrossRef]
- Mao, J.; Kamiya, Y.; Okuhara, T. Alkylation of 1,3,5-trimethylbenzene with γ-butyrolactone over heteropolyacid catalysts. Appl. Catal. A Gen. 2003, 255, 337–344. [Google Scholar] [CrossRef]
- Zain, G.; Bondarev, D.; Doháňošová, J.; Mosnáček, J. Oxygen-Tolerant Photochemically Induced Atom Transfer Radical Polymerization of the Renewable Monomer Tulipalin A. ChemPhotoChem 2019, 3, 1138–1145. [Google Scholar] [CrossRef]
- Shen, Y.; Xiong, W.; Li, Y.; Zhao, Z.; Lu, H.; Li, Z. Chemoselective Polymerization of Fully Biorenewable α-Methylene-γ-Butyrolactone Using Organophosphazene/Urea Binary Catalysts Toward Sustainable Polyesters. CCS Chem. 2021, 3, 620–630. [Google Scholar] [CrossRef]
- Trumbo, D.L. The copolymerization of substituted styrenes with α-methylene-γ-butyrolactone. Polym. Bull. 1995, 35, 265–269. [Google Scholar] [CrossRef]
- Feng, R.; Jie, S.; Braunstein, P.; Li, B.G. Gradient copolymers of ε-caprolactone and δ-valerolactone via solvent-free ring-opening copolymerization with a pyridyl-urea/MTBD system. J. Polym. Sci. 2020, 58, 2108–2115. [Google Scholar] [CrossRef]
- Hua, Q.; Jiea, S.Y.; Braunsteinb, P.; Lia, B.G. Ring-opening Copolymerization of ε-Caprolactone and δ-Valerolactone Catalyzed by a 2,6-Bis(amino)phenol Zinc Complex. Chin. J. Polym. Sci. 2020, 38, 240–247. [Google Scholar] [CrossRef]
- Lin, W. Comparison of thermal characteristics and degradation properties of ε-caprolactone copolymers. J. Biomed. Mater. Res. 1999, 47, 420–423. [Google Scholar] [CrossRef]
- Fernández, J.; Larrañaga, A.; Etxeberria, A.; Sarasua, J. Tensile behavior and dynamic mechanical analysis of novel poly(lactide/δ-valerolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2014, 35, 39–50. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; Zhang, L. Binary catalytic system for homo- and block copolymerization of ε-caprolactone with δ-valerolactone. RSC Adv. 2020, 10, 25979–25987. [Google Scholar] [CrossRef]
- Mahhaa, Y.; Atlamsani, A.; Blais, J.C.; Tessier, M.; Bregault, J.M.; Salles, L. Oligomerization of ε-caprolactone and δ-valerolactone using heteropolyacid initiators and vanadium or molybdenum complexes. J. Mol. Catal. A Chem. 2005, 234, 63–73. [Google Scholar] [CrossRef]
- Gagliardi, M.; Di Michele, F.; Mazzolai, B.; Bifone, A. Chemical synthesis of a biodegradable PEGylated copolymer from ε-caprolactone and γ-valerolactone: Evaluation of reaction and functional properties. J. Polym. Res. 2015, 22, 17. [Google Scholar] [CrossRef]
- Gagliardi, M.; Bifone, A. Ring- opening copolymerization thermodynamics and kinetics of γ-valerolactone/ε-caprolactone. PLoS ONE 2018, 13, e0199231. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Schneiderman, D.K.; Bates, F.S.; Zhang, K. Poly [Beta-Methyl-Delta-Valerolactone] Block Polymers. WO Patent 2015/161169A1, 22 October 2015. [Google Scholar]
- Naumann, S.; Scholten, P.B.V.; Wilson, J.A.; Dove, A.P. Dual Catalysis for Selective Ring-Opening Polymerization of Lactones: Evolution toward Simplicity. J. Am. Chem. Soc. 2015, 137, 14439–14445. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, G.; Parzuchowski, P. ROP of Cyclic Carbonates and ROP of Macrocycles—Latest Developments. Ref. Modul. Mater. Sci. Mater. Eng. 2016. [Google Scholar] [CrossRef]
- Pêgo, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Copolymers of trimethylene carbonate and ε-caprolactone for porous nerve guides: Synthesis and properties. J. Biomater. Sci. Polym. Ed. 2001, 12, 35–53. [Google Scholar] [CrossRef]
- Couffin, A.; Delcroix, D.; Martin-Vaca, B.; Bourissou, D.; Navarro, C. Mild and Efficient Preparation of Block and Gradient Copolymers by Methanesulfonic Acid Catalyzed Ring-Opening Polymerization of Caprolactone and Trimethylene Carbonate. Macromolecules 2013, 46, 4354–4360. [Google Scholar] [CrossRef]
- Olsen, P.; Odelius, K.; Keul, H.; Albertsson, A.C. Macromolecular Design via an Organocatalytic, Monomer-Specific and Temperature-Dependent “On/Off Switch”. High Precision Synthesis of Polyester/Polycarbonate Multiblock Copolymers. Macromolecules 2015, 48, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.; Samad, A.A.; Jundi, A.E.; Bethry, A.; Bakkour, Y.; Coudane, J.; Nottelet, B. Stabilization of poly (ethylene glycol)-poly (ε-caprolactone) star block copolymer micelles via aromatic groups for improved drug delivery properties. J. Colloid Interface Sci. 2017, 514, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, B.K.; Park, S.H.; Kim, M.G.; Lee, J.W.; Lee, H.Y.; Lee, H.B.; Kim, J.H.; Kim, M.S. Preparation of Biodegradable and Elastic Poly (ε-caprolactone-co-lactide) Copolymers and Evaluation as a Localized and Sustained Drug Delivery Carrier. Int. J. Mol. Sci. 2017, 18, 671. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, K.T.; Collins, H.G.; Balija, A.M. Biorenewable triblock copolymers consisting of l-lactide and ε-caprolactone for removing organic pollutants from water: A lifecycle neutral solution. BMC Chem. 2019, 13, 122–130. [Google Scholar] [CrossRef]
- Saat, M.N.; Suffian, M.; Annuar, M. One-pot lipase-catalyzed esterification of ε-caprolactone with methyl-D-glucopyranoside and its elongation with free 6-hydroxyhexanoate monomer units. Biotechnol. Appl. Biochem. 2019, 67, 354–365. [Google Scholar] [CrossRef]
- Imsombut, T.; Srisa-Ard, M.; Baimark, Y. Synthesis of Star-Shaped ε-Caprolactone Oligomers for use as Plasticizers of Poly (L-Lactide) Bioplastic Films. Orient. J. Chem. 2017, 33, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Purcell, M.; Steele, J.A.M.; Lee, K.-Y.; McCullen, S.; Shakesheff, K.M.; Bismarck, A.; Stevens, M.M.; Howdle, S.M.; Williams, C.K. Porous Copolymers of ε-Caprolactone as Scaffolds for Tissue Engineering. Macromolecues 2013, 46, 8136–8143. [Google Scholar] [CrossRef]
- Todea, A.; Bîtcan, I.; Aparaschivei, D.; Păușescu, I.; Badea, V.; Péter, F.; Gherman, V.D.; Rusu, G.; Nagy, L.; Kéki, S.; et al. Biodegradable Oligoesters of ε-Caprolactone and 5-Hydroxymethyl-2-Furancarboxylic Acid Synthesized by Immobilized Lipases. Polymers 2019, 11, 1402. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Guo, C.; Dou, H.; Zuo, Y.; Sun, Y.; Zhang, J.; Li, W. Tailoring the degradation and mechanical properties of poly(ε-caprolactone) incorporating functional ε-caprolactone-based copolymers. Polym. Chem. 2019, 10, 3786–3796. [Google Scholar] [CrossRef]
- Martello, M.T.; Burns, A.; Hillmyer, M. Bulk Ring-Opening Transesterification Polymerization of the Renewable δ-Decalactone Using an Organocatalyst. ACS Macro Lett. 2011, 1, 131–135. [Google Scholar] [CrossRef]
- Fernández, J.; Etxeberria, A.; Varga, A.L.; Sarasua, J.-R. Synthesis and characterization of ω-pentadecalactone-co-ε-decalactone copolymers: Evaluation of thermal, mechanical and biodegradation properties. Poly-mers 2015, 81, 12–22. [Google Scholar] [CrossRef]
- Olsén, P.; Borke, T.; Odelius, K.; Albertsson, A.C. ε-Decalactone: A Thermoresilient and Toughening Comonomer to Poly(L-lactide). Biomacromolecules 2013, 14, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Thongkham, S.; Monot, J.; Martin-Vaca, B.; Bourissou, D. Simple In-Based Dual Catalyst Enables Significant Progress in ε-Decalactone Ring-Opening (Co)polymerization. Macromolecues 2019, 52, 8103–8113. [Google Scholar] [CrossRef]
- Martello, M.T.; Schneiderman, D.K.; Hillmyer, M.A. Synthesis and Melt Processing of Sustainable Poly (ε-decalactone)-block-Poly (lactide) Multiblock Thermoplastic Elastomers. ACS Sustain. Chem. Eng. 2014, 2, 2519–2526. [Google Scholar] [CrossRef]
- Hakala, R.A.; Korhonen, H.; Holappa, S.; Seppälä, J.V. Hydrophobicities of poly(ε-caprolactone) oligomers functionalized with different succinic anhydrides. Eur. Polym. J. 2009, 45, 557–564. [Google Scholar] [CrossRef]














| Monomer | Comonomer(s) | Product | Initiator or Catalyst | Source |
|---|---|---|---|---|
| β-propiolactone | Valine | Poly(VAL-co-β-PL) | NaOH/MeOH | [64] |
| – | Poly-β-propiolactone | Temperature | [65,66] | |
| FeCl3 | [65] | |||
| H2 SO4 | ||||
| γ-butyrolactone | Poly(γ-BL-co-β-PL) | AlEt3-H2 O | [67] | |
| Lactide | Poly(LA-co-β-PL) | TfOH/MeOH | [68] | |
| Glycolide | Poly(G-co-β-PL) | Anionic catalyst | [69] | |
| Acidic catalyst | ||||
| Complexing catalyst | ||||
| Acetonitrile | Poly(AN-co-β-PL) | α, α’-azobisisobutyronitrile benzoylperoxide | [70] | |
| Styrene | Poly(S-co-β-PL) | |||
| 2-oxazoline | Poly(OXZ-co-β-PL) | Temperature | [71] | |
| PEG | Poly(EG-co-β-PL) | Temperature | [72] | |
| β-butyrolactone | ε-caprolactone | Poly(β-BL-co-ε-CL) | NaH | [41] |
| – | PHB | NaH | [44] | |
| Sodium phenoxides | [40] | |||
| Aluminum complexes | [73] | |||
| ε-decalactone | Poly(β-BL-co-ε-DL) | Y (III) complexes | [42] | |
| β-malolactone | Poly(β-BL-co-β-ML) | Metal catalyst/Isopropanol | [45] | |
| Lactide | Poly(LA-co-β-BL) | Y (III) complexes | [43] | |
| ε-caprolactone, PEG | Poly(ε-CL-co-β-BL-co-EG) | Trifluoromethanesulfonate sulfone acid | [74] | |
| γ-valerolactone | Poly(β-BL-co-γ-VL) | BF3-OEt2 | [75] | |
| γ-butyrolactone | ε-caprolactone, Ethylene oxide | Poly(EO-co-ε-CL-co- γ-BL) | TBD | [48] |
| – | Poly-γ-butyrolactone | Zeolites | [76] | |
| PEG | Poly(EG-co-γ-BL) | CTPB/m PEG | [46] | |
| Lactide | Poly(LA-co-γ-BL) | CTPB | [47] | |
| α-methylene-γ-butyrolactone | – | PMBL | Cu/( photoATRP ) | [77] |
| Benzyl alcohol/urea | [78] | |||
| Styrene | Poly(S-co-α-M-γ-BL) | AIBN | [79] | |
| δ-valerolactone | ε-caprolactone | Poly(δ-VL-co-ε-CL) | Pyridyl urea/MTBD | [80] |
| Zinc 2,6-bisaminophenol complex | [81] | |||
| Temperature | [82] | |||
| Lactide | Poly(LA-co-δ-VL) | Bismuth sub-sallycinate (III) ( BiSS ) | [83] | |
| Tin octanoate (SnOct2 ) | [49,83] | |||
| ε-caprolactone,PEG | Poly(δ-VL-co-ε-CL- co- EG) | ITU/YCl3 (2,3,6,7-tetrahydro- 5H-thiazolo [3,2-a] pyrimidine) | [84] | |
| – | Oligo(δ-VL) | H5 [PMo12−2V2 O40 ] · aq / MeOH | [85] | |
| γ-valerolactone | ε-caprolactone, mPEG | Poly(mEG-co-ε-CL- co-γ-VL) | Tin octanoate (SnOct2 ) | [86] |
| ε-caprolactone | Poly(ε-CL-co-γ-VL) | mPEG/octanoate, tin (SnOct2) | [87] | |
| Temperature | [82] | |||
| δ-caprolactone | ω-pentadecalactone | Poly(ω-PDL-co-δ-CL) | Triphenyl bismuth (Ph3Bi) | [60] |
| – | Poly-δ-caprolactone | TBD/Benzyl Alcohol | [88] | |
| ε-caprolactone | – | Poly-ε-caprolactone | 4-dimethylaminopyridine (DMAP), Lewis acid/Benzyl alcohol | [89] |
| γ-butyrolactone | Poly(ε-CL-co-γ-BL) | Heat | [82] | |
| γ-caprolactone | Poly(ε-CL-co-γ-CL) | |||
| Trimethylene carbonate | Poly(ε-CL-co-TMC) | Tin octanoate (SnOct2 ) | [90,91] | |
| Methanesulfonic acid | [92] | |||
| Carbonate, 2-allyloxymethyl-2-ethyl-trimethylene (AOMEC) | Poly(ε-CL-co- AOMEC) | 1,5,7-triazabicyclo [4.4.0] dec-5-ene(TBD) | [93] | |
| PEG | Poly(ε-CL-co-EG) | PEG(NH2 )8/octoate, tin (SnOct2 ) | [94] | |
| Tin octanoate (SnOct2 ) | [51] | |||
| Lactide | Poly(LA-co-ε-CL) | mPEG/octanoate, tin (SnOct2) | [95] | |
| 1,4-benzenedimethanol / octoate, tin(SnOct2 ) | [96] | |||
| Methyl-δ-glucopyranoside | Oligo(MGP-co-ε-CL) | Immozyme CALB | [97] | |
| – | Oligo-ε-caprolactone | Tin octanoate (SnOct2 )/Boltorn H2004 | [98] | |
| CALB | [19] | |||
| Acetic acid 5-acetoxy-6-oxotetrahydropyran-2-yl methyl ester | Poly(AOTME-co-ε-CL) | Tin butoxide (Sn(Obu)2 ) | [99] | |
| 5-hydroxymethyl-2-furancarboxylic acid | Oligo(HMFA-co-ε-CL) | CALB | [100] | |
| Polyhexamethylene guanidine (PHMG) | Poly(HMG-co-ε-CL) | PHMG | [52] | |
| γ-(carbamic acid benzyl ester)-ε-caprolactone (CABCL) | Poly(γ-CABCL-co-ε-CL) | mPEG / octanoate, tin (SnOct2) | [101] | |
| δ-decalactone | PEG | Poly(EG-co-δ-DL) | PEG/1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) | [61] |
| mPEG | Poly(mEG-co-δ-DL) | mPEG/1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) | ||
| – | Poly-δ-decalactone | triazabicyclo [4.4.0] dec-5-ene (TBD) / 1,4-benzenedimethanol (BDM) | [102] | |
| ε-decalactone | ω-pentadecalactone | Poly(ω-PDL-co-ε-DL) | Triphenyl bismuth (Ph3 Bi) | [103] |
| – | Poly-ε-decalactone | triazabicyclo [4.4.0] dec-5-ene (TBD)/benzyl alcohol | [104] | |
| Tin octanoate (SnOct2)/Benzyl alcohol | [104] | |||
| InCl3 / Triethylamine (NEt3 ) / Benzyl alcohol | [105] | |||
| InCl3 / Triethylamine (NEt3 ) / benzyl amine | [105] | |||
| Lactide | Poly(LA-co-ε-DL) | Tin octanoate (SnOct2 ) / 1,4-butanediol | [104] | |
| Tin octanoate (SnOct2 ) / 1,4-benzenedimethanol (BDM) | [106] | |||
| ε-caprolactone | Poly(ε-CL-co-ε-DL) | InCl3 / Dimethoxybenzyl Alcohol (DMBA), Triethylamine (NEt3) | [105] | |
| Tin octanoate (SnOct2)/1,4-benzenedimethanol (BDM) | [62] | |||
| mPEG | Poly(mEG-co-ε-DL) | mPEG | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bińczak, J.; Dziuba, K.; Chrobok, A. Recent Developments in Lactone Monomers and Polymer Synthesis and Application. Materials 2021, 14, 2881. https://doi.org/10.3390/ma14112881
Bińczak J, Dziuba K, Chrobok A. Recent Developments in Lactone Monomers and Polymer Synthesis and Application. Materials. 2021; 14(11):2881. https://doi.org/10.3390/ma14112881
Chicago/Turabian StyleBińczak, Jakub, Krzysztof Dziuba, and Anna Chrobok. 2021. "Recent Developments in Lactone Monomers and Polymer Synthesis and Application" Materials 14, no. 11: 2881. https://doi.org/10.3390/ma14112881
APA StyleBińczak, J., Dziuba, K., & Chrobok, A. (2021). Recent Developments in Lactone Monomers and Polymer Synthesis and Application. Materials, 14(11), 2881. https://doi.org/10.3390/ma14112881