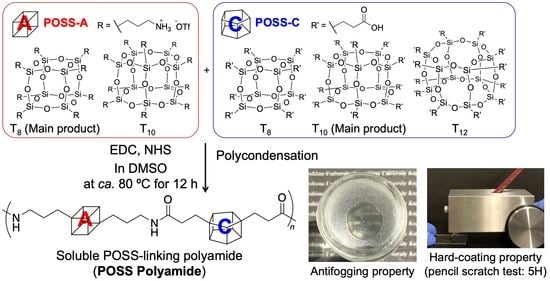
Preparation of Soluble POSS-Linking Polyamide and Its Application in Antifogging Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of POSS-A
2.3. Preparation of Carboxyl-Functionalized Rod-Like Polysilsesquioxane as a Precursor of POSS-C
2.4. Preparation of POSS-C
2.5. Preparation of POSS Polyamide
2.6. Preparation of the Cast Film of POSS Polyamide
2.7. Measurements
3. Results and Discussion
3.1. Preparation and Characterizations of POSS Polyamide
3.2. Self-Standing Film Formability of POSS Polyamide
3.3. Thermal Properties of POSS Polyamide
3.4. Hard-Coating and Antifogging Properties of a POSS Polyamide Cast Film
3.5. Effect of the Heat-Treatment Temperature on the Antifogging and Hard-Coating Properties of POSS Polyamide Cast Film
3.6. Antifogging and Hard-Coating Properties of Cast Films of the Starting Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Gong, X. Special oleophobic and hydrophilic surfaces: Approaches, mechanisms, and applications. J. Mater. Chem. A 2017, 5, 3759–3773. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.; Ming, W. Antifogging and Frost-Resisting Polymeric Surfaces. Adv. Polym. Sci. 2018, 284, 185–214. [Google Scholar] [CrossRef]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The Dry-Style Antifogging Properties of Mosquito Compound Eyes and Artificial Analogues Prepared by Soft Lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J.H.; Dou, S.X. Fly-Eye Inspired Superhydrophobic Anti-Fogging Inorganic Nanostructures. Small 2014, 10, 3001–3006. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Photogeneration of highly amphiphilic TiO2 surfaces. Adv. Mater. 1998, 10, 135–138. [Google Scholar] [CrossRef]
- Sun, R.-D.; Nakajima, A.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Photoinduced Surface Wettability Conversion of ZnO and TiO2 Thin Films. J. Phys. Chem. B 2001, 105, 1984–1990. [Google Scholar] [CrossRef]
- Zhang, X.-T.; Sato, O.; Taguchi, M.; Einaga, Y.; Murakami, T.; Fujishima, A. Self-Cleaning Particle Coating with Antireflection Properties. Chem. Mater. 2005, 17, 696–700. [Google Scholar] [CrossRef]
- Lee, D.; Rubner, M.F.; Cohen, R.E. All-Nanoparticle Thin-Film Coatings. Nano Lett. 2006, 6, 2305–2312. [Google Scholar] [CrossRef]
- Tricoli, A.; Righettoni, M.; Pratsinis, S.E. Anti-Fogging Nanofibrous SiO2 and Nanostructured SiO2−TiO2 Films Made by Rapid Flame Deposition and In Situ Annealing. Langmuir 2009, 25, 12578–12584. [Google Scholar] [CrossRef]
- Chemin, J.-B.; Bulou, S.; Baba, K.; Fontaine, C.; Sindzingre, T.; Boscher, N.D.; Choquet, P. Transparent anti-fogging and self-cleaning TiO2/SiO2 thin films on polymer substrates using atmospheric plasma. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Y.; Song, C.; Li, Y.; Jiang, B. A simple sol-gel method to prepare superhydrophilic silica coatings. Mater. Lett. 2017, 188, 316–318. [Google Scholar] [CrossRef]
- Zhao, J.; Meyer, A.; Ma, L.; Ming, W. Acrylic coatings with surprising antifogging and frost-resisting properties. Chem. Commun. 2013, 49, 11764–11766. [Google Scholar] [CrossRef]
- Lee, H.; Alcaraz, M.L.; Rubner, M.F.; Cohen, R.E. Zwitter-Wettability and Antifogging Coatings with Frost-Resisting Capabilities. ACS Nano 2013, 7, 2172–2185. [Google Scholar] [CrossRef]
- Zhao, J.; Meyer, A.; Ma, L.; Wang, X.; Ming, W. Terpolymer-based SIPN coating with excellent antifogging and frost-resisting properties. RSC Adv. 2015, 5, 102560–102566. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.; Zhang, R.; Li, C.; Zhu, K.; Sun, P.; Zhao, Y.; Ren, L.; Yuan, X. Enhancing antifogging/frost-resisting performances of amphiphilic coatings via cationic, zwitterionic or anionic polyelectrolytes. Chem. Eng. J. 2019, 357, 667–677. [Google Scholar] [CrossRef]
- Zhang, T.; Fang, L.; Lin, N.; Wang, J.; Wang, Y.; Wu, T.; Song, P. Highly transparent, healable, and durable anti-fogging coating by combining hydrophilic pectin and tannic acid with poly(ethylene terephthalate). Green Chem. 2019, 21, 5405–5413. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, P.; Song, L.; Tian, L.; Ming, W.; Ren, L. Highly efficient antifogging and frost-resisting acrylic coatings from one-step thermal curing. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124160. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Self-Cleaning and Next Generation Anti-Fog Surfaces and Coatings. Macromol. Rapid Commun. 2008, 29, 455–466. [Google Scholar] [CrossRef]
- Yao, B.; Zhao, H.; Wang, L.; Liu, Y.; Zheng, C.; Li, H.; Sun, C. Synthesis of acrylate-based UV/thermal dual-cure coatings for antifogging. J. Coat. Technol. Res. 2017, 15, 149–158. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Loy, D.A.; Baugher, B.M.; Baugher, C.R.; Schneider, D.A.; Rahimian, K. Substituent effects on the sol-gel chemistry of organotrialkoxysilanes. Chem. Mater. 2000, 12, 3624–3632. [Google Scholar] [CrossRef] [Green Version]
- Cordes, D.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Laine, R.M.; Roll, M.F. Polyhedral Phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; He, C. Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Kaneko, Y.; Toyodome, H.; Shoiriki, M.; Iyi, N. Preparation of ionic silsesquioxanes with regular structures and their hybridization. Int. J. Polym. Sci. 2012, 2012, 684278. [Google Scholar] [CrossRef]
- Kaneko, Y. Ionic silsesquioxanes: Preparation, structure control, characterization, and applications. Polymer 2018, 144, 205–224. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Adachi, S.; Chujo, Y. Structure-property relationship of octa-substituted POSS in thermal and mechanical reinforcements of conventional polymers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5690–5697. [Google Scholar] [CrossRef]
- Wu, S.; Hayakawa, T.; Kikuchi, R.; Grunzinger, A.S.J.; Kakimoto, M.-A.; Oikawa, H. Synthesis and Characterization of Semiaromatic Polyimides Containing POSS in Main Chain Derived from Double-Decker-Shaped Silsesquioxane. Macromolecules 2007, 40, 5698–5705. [Google Scholar] [CrossRef]
- Wu, S.; Hayakawa, T.; Kakimoto, M.-A.; Oikawa, H. Synthesis and Characterization of Organosoluble Aromatic Polyimides Containing POSS in Main Chain Derived from Double-Decker-Shaped Silsesquioxane. Macromolecules 2008, 41, 3481–3487. [Google Scholar] [CrossRef]
- Jung, J.H.; Laine, R.M. Beads on a Chain (BOC) Polymers Formed from the Reaction of NH2PhSiO1.5]x[PhSiO1.5]10–x and [NH2PhSiO1.5]x[PhSiO1.5]12–x Mixtures (x= 2–4) with the Diglycidyl Ether of Bisphenol A. Macromolecules 2011, 44, 7263–7272. [Google Scholar] [CrossRef]
- Yoshimatsu, M.; Komori, K.; Ohnagamitsu, Y.; Sueyoshi, N.; Kawashima, N.; Chinen, S.; Murakami, Y.; Izumi, J.; Inoki, D.; Sakai, K.; et al. Necklace-shaped dimethylsiloxane polymers bearing a polyhedral oli-gomeric silsesquioxane cage prepared by polycondensation and ring-opening polymerization. Chem. Lett. 2012, 41, 622–624. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.; Zheng, S. Organic–inorganic polyurethanes with 3,13-dihydroxypropyloctaphenyl double-decker silsesquioxane chain extender. Polym. Chem. 2012, 4, 1491–1501. [Google Scholar] [CrossRef]
- Maegawa, T.; Irie, Y.; Fueno, H.; Tanaka, K.; Naka, K. Synthesis and polymerization of a para-disubstituted T8-caged hexaisobutyl-POSS monomer. Chem. Lett. 2014, 43, 1532–1534. [Google Scholar] [CrossRef]
- Maegawa, T.; Irie, Y.; Imoto, H.; Fueno, H.; Tanaka, K.; Naka, K. para-Bisvinylhexaisobutyl-substituted T 8 caged monomer: Synthesis and hydrosilylation polymerization. Polym. Chem. 2015, 6, 7500–7504. [Google Scholar] [CrossRef]
- Tokunaga, T.; Koge, S.; Mizumo, T.; Ohshita, J.; Kaneko, Y. Facile preparation of a soluble polymer containing polyhedral oligomeric silsesquioxane units in its main chain. Polym. Chem. 2015, 6, 3039–3045. [Google Scholar] [CrossRef]
- Kaneko, Y.; Shoiriki, M.; Mizumo, T. Preparation of cage-like octa(3-aminopropyl)silsesquioxane trifluoromethanesulfonate in higher yield with a shorter reaction time. J. Mater. Chem. 2012, 22, 14475–14478. [Google Scholar] [CrossRef]
- Tokunaga, T.; Shoiriki, M.; Mizumo, T.; Kaneko, Y. Preparation of low-crystalline POSS containing two types of alkylammonium groups and its optically transparent film. J. Mater. Chem. C 2014, 2, 2496–2501. [Google Scholar] [CrossRef]
- Imai, K.; Kaneko, Y. Preparation of Ammonium-Functionalized Polyhedral Oligomeric Silsesquioxanes with High Proportions of Cagelike Decamer and Their Facile Separation. Inorg. Chem. 2017, 56, 4133–4140. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kaneko, Y. Selective and high-yielding preparation of ammonium-functionalized cage-like octasilsesquioxanes using superacid catalyst in dimethyl sulfoxide. Chem. Lett. 2018, 47, 864–867. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kaneko, Y. Effect of Reaction Temperature and Time on the Preferential Preparation of Cage Octamer and Decamer of Ammonium-Functionalized POSSs. Bull. Chem. Soc. Jpn. 2019, 92, 1060–1067. [Google Scholar] [CrossRef]
- Hasebe, R.; Kaneko, Y. Control of Crystalline-Amorphous Structures of Polyhedral Oligomeric Silsesquioxanes Containing Two Types of Ammonium Side-Chain Groups and Their Properties as Protic Ionic Liquids. Molecules 2019, 24, 4553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuma, T.; Kaneko, Y. Preparation of carboxyl-functionalized polyhedral oligomeric silsesquioxane by a structural transformation reaction from soluble rod-like polysilsesquioxane. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2511–2518. [Google Scholar] [CrossRef]
- Toyodome, H.; Kaneko, Y.; Shikinaka, K.; Iyi, N. Preparation of carboxylate group-containing rod-like polysilsesquioxane with hexagonally stacked structure by sol–gel reaction of 2-cyanoethyltriethoxysilane. Polymer 2012, 53, 6021–6026. [Google Scholar] [CrossRef]
- Park, E.S.; Ro, H.W.; Nguyen, C.V.; Jaffe, R.L.; Yoon, D.Y. Infrared Spectroscopy Study of Microstructures of Poly(silsesquioxane)s. Chem. Mater. 2008, 20, 1548–1554. [Google Scholar] [CrossRef]
- Chimjarn, S.; Kunthom, R.; Chancharone, P.; Sodkhomkhum, R.; Sangtrirutnugul, P.; Ervithayasuporn, V. Synthesis of aromatic functionalized cage-rearranged silsesquioxanes (T8, T10, and T12) via nucleophilic substitution reactions. Dalton Trans. 2015, 44, 916–919. [Google Scholar] [CrossRef]













| Compound | Solubility 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | DMSO 2 | DMF 2 | MeOH 2 | Me2CO 2 | AcOEt 2 | CHCl3 2 | Toluene | Hexane | |
| POSS-A | + | + | + | + | − | − | − | − | − |
| POSS-A (Counterion: Cl−) | + | + | − | + | − | − | − | − | − |
| POSS-C | + | + | + | + | + | − | − | − | − |
| POSS polyamide | + | + | + | + | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozuma, T.; Mihata, A.; Kaneko, Y. Preparation of Soluble POSS-Linking Polyamide and Its Application in Antifogging Films. Materials 2021, 14, 3178. https://doi.org/10.3390/ma14123178
Kozuma T, Mihata A, Kaneko Y. Preparation of Soluble POSS-Linking Polyamide and Its Application in Antifogging Films. Materials. 2021; 14(12):3178. https://doi.org/10.3390/ma14123178
Chicago/Turabian StyleKozuma, Tomoya, Aki Mihata, and Yoshiro Kaneko. 2021. "Preparation of Soluble POSS-Linking Polyamide and Its Application in Antifogging Films" Materials 14, no. 12: 3178. https://doi.org/10.3390/ma14123178
APA StyleKozuma, T., Mihata, A., & Kaneko, Y. (2021). Preparation of Soluble POSS-Linking Polyamide and Its Application in Antifogging Films. Materials, 14(12), 3178. https://doi.org/10.3390/ma14123178