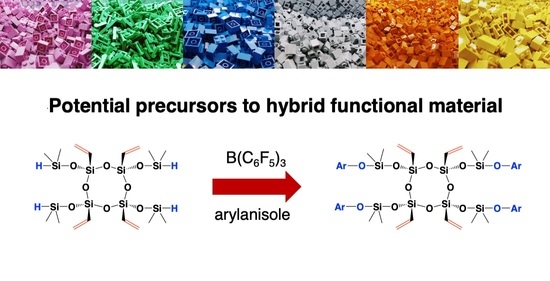
Vinyl-Functionalized Janus Ring Siloxane: Potential Precursors to Hybrid Functional Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Potassium All-Cis-Tetravinylcyclotetrasiloxanolate
2.3. Synthesis of Hydrido-Functionalized Janus Precursor [ViSi(OSiMe2H)O]4
2.4. Synthesis of Vinyl-Functionalized Janus Rings [ViSi(OSiMe2OR)O]4
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, K.; Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M. Fabrication of superhydrophobic coatings with self-cleaning properties on cotton fabric based on Octa vinyl polyhedral oligomeric silsesquioxane/polydimethylsiloxane (OV-POSS/PDMS) nanocomposite. J. Colloid Interface Sci. 2019, 540, 78–87. [Google Scholar] [CrossRef]
- Chan, K.L.; Sonar, P.; Sellinger, A. Cubic silsesquioxanes for use in solution processable organic light emitting diodes (OLED). J. Mater. Chem. 2009, 19, 9103–9120. [Google Scholar] [CrossRef]
- Wang, D.; Xue, L.; Li, L.; Deng, B.; Feng, S.; Liu, H.; Zhao, X. Rational Design and Synthesis of Hybrid Porous Polymers Derived from Polyhedral Oligomeric Silsesquioxanes via Heck Coupling Reactions. Macromol. Rapid Commun. 2013, 34, 861–866. [Google Scholar] [CrossRef]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Hanprasit, S.; Masik, M.; Prigyai, N.; Kiatkamjornwong, S. Silsesquioxane cages as fluoride sensors. Chem. Commun. 2017, 53, 12108–12111. [Google Scholar] [CrossRef]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Boonkitti, P.; Phuekphong, A.; Prigyai, N.; Kladsomboon, S.; Kiatkamjornwong, S. Anion identification using silsesquioxane cages. Chem. Sci. 2018, 9, 7753–7765. [Google Scholar] [CrossRef] [Green Version]
- Prigyai, N.; Chanmungkalakul, S.; Thanyalax, S.; Sukwattanasinitt, M.; Ervithayasuporn, V. Cyclic siloxanes conjugated with fluorescent aromatic compounds as fluoride sensors. Mater. Adv. 2020, 1, 3358–3368. [Google Scholar] [CrossRef]
- Żak, P.; Pietraszuk, C. Application of olefin metathesis in the synthesis of functionalized polyhedral oligomeric silsesquioxanes (POSS) and POSS-containing polymeric materials. Beilstein J. Org. Chem. 2019, 15, 310–332. [Google Scholar] [CrossRef] [PubMed]
- Itami, Y.; Marciniec, B.; Kubicki, M. Functionalization of Octavinylsilsesquioxane by Ruthenium-Catalyzed Silylative Coupling versus Cross-Metathesis. Chem. A Eur. J. 2004, 10, 1239–1248. [Google Scholar] [CrossRef]
- Grzelak, M.; Januszewski, R.; Marciniec, B. Synthesis and Hydrosilylation of Vinyl-Substituted Open-Cage Silsesquioxanes with Phenylsilanes: Regioselective Synthesis of Trifunctional Silsesquioxanes. Inorg. Chem. 2020, 59, 7830–7840. [Google Scholar] [CrossRef]
- Scholder, P.; Nischang, I. Miniaturized catalysis: Monolithic, highly porous, large surface area capillary flow reactors con-structed in situ from polyhedral oligomeric silsesquioxanes (POSS). Catal. Sci. Technol. 2015, 5, 3917–3921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, F.; Nischang, I. Tailor-Made Hybrid Organic–Inorganic Porous Materials Based on Polyhedral Oligomeric Silsesqui-oxanes (POSS) by the Step-Growth Mechanism of Thiol-Ene “Click” Chemistry. Chem. Eur. J. 2013, 19, 17310–17313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, K.; Su, H.; Li, X.; Feng, X.; Wang, Z.; Zhang, W.; Zhu, S.; Wesdemiotis, C.; Cheng, S.Z.D.; et al. Tuning “thiol-ene” reactions toward controlled symmetry breaking in polyhedral oligomeric silsesquioxanes. Chem. Sci. 2014, 5, 1046–1053. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.-H.; Guo, K.; Wang, Z.; Chen, Z.; Wesdemiotis, C.; Quirk, R.P.; Zhang, W.-B.; Cheng, S.Z.D. Synthesis of Shape Amphiphiles Based on POSS Tethered with Two Symmetric/Asymmetric Polymer Tails via Sequential “Grafting-from” and Thiol–Ene “Click” Chemistry. ACS Macro Lett. 2012, 1, 834–839. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H., Jr.; Pittman, C.U. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. Mater. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Sangtrirutnugul, P.; Chaiprasert, T.; Hunsiri, W.; Jitjaroendee, T.; Songkhum, P.; Laohhasurayotin, K.; Osotchan, T.; Ervithayasuporn, V. Tunable Porosity of Cross-Linked-Polyhedral Oligomeric Silsesquioxane Supports for Palladium-Catalyzed Aerobic Alcohol Oxidation in Water. ACS Appl. Mater. Interfaces 2017, 9, 12812–12822. [Google Scholar] [CrossRef] [PubMed]
- Dudziec, B.; Żak, P.; Marciniec, B. Synthetic Routes to Silsesquioxane-Based Systems as Photoactive Materials and Their Precursors. Polymers 2019, 11, 504. [Google Scholar] [CrossRef] [Green Version]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Feher, F.J.; Newman, D.A.; Walzer, J.F. Silsesquioxanes as models for silica surfaces. J. Am. Chem. Soc. 1989, 111, 1741–1748. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fu, Y.; Liu, S. Polyhedral Oligomeric Silsesquioxane (POSS)-Modified Thermoplastic and Thermosetting Nanocom-posites: A Review. Polym. Polym. Compos. 2008, 16, 483–500. [Google Scholar]
- Dong, F.; Lu, L.; Ha, C.-S. Silsesquioxane-Containing Hybrid Nanomaterials: Fascinating Platforms for Advanced Applica-tions. Macromol. Chem. Phys. 2019, 220, 1800324. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Zhao, X.; Jiang, P.; Yu, H. Flame retardancy and thermal stability of polyhedral oligomeric silsesquioxane nanocomposites. Fire Mater. 2011, 37, 1–16. [Google Scholar] [CrossRef]
- Scapini, P.; Figueroa, C.A.; Amorim, C.L.; Machado, G.; Mauler, R.S.; Crespo, J.S.; Oliveira, R.V. Thermal and morphological properties of high-density polyethylene/ethylene–vinyl acetate copolymer composites with polyhedral oligomeric silsesquiox-ane nanostructure. Polym. Int. 2010, 59, 175–180. [Google Scholar]
- Endo, H.; Takeda, N.; Takanashi, M.; Imai, T.; Unno, M. Refractive Indices of Silsesquioxanes with Various Structures. Silicon 2014, 7, 127–132. [Google Scholar] [CrossRef]
- Endo, H.; Takeda, N.; Unno, M. Synthesis and Properties of Phenylsilsesquioxanes with Ladder and Double-Decker Structures. Organometallics 2014, 33, 4148–4151. [Google Scholar] [CrossRef]
- Unno, M.; Endo, H.; Takeda, N. Synthesis and Structures of Extended Cyclic Siloxanes. Heteroat. Chem. 2014, 25, 525–532. [Google Scholar] [CrossRef]
- Endo, H.; Takeda, N.; Unno, M. Stereoisomerization of Cyclic Silanols. Chem. Asian J. 2017, 12, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Panisch, R.; Bassindale, A.R.; Korlyukov, A.A.; Pitak, M.B.; Coles, S.J.; Taylor, P.G. Selective Derivatization and Charac-terization of Bifunctional “Janus-Type” Cyclotetrasiloxanes. Organometallics 2013, 32, 1732–1742. [Google Scholar] [CrossRef]
- Yagihashi, F.; Igarashi, M.; Nakajima, Y.; Sato, K.; Yumoto, Y.; Matsui, C.; Shimada, S. Unexpected Selectivity in Cyclotetra-siloxane Formation by the Hydrolytic Condensation Reaction of Trichloro(phenyl)silane. Eur. J. Inorg. Chem. 2016, 2016, 2882–2886. [Google Scholar] [CrossRef]
- Ryuichi, I.; Yuriko, K.; Yusuke, K. Cyclic Tetrasiloxanetetraols: Formation, Isolation, and Characterization. Chem. Lett. 2009, 38, 364–365. [Google Scholar]
- Shankar, R.; Chaudhary, M.; Molloy, K.C.; Kociok-Köhn, G. New cyclotetrasiloxanes bearing sila-alkyl substituted side chains and their applications as templates for gold nanowires. Dalton Trans. 2013, 42, 7768–7774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, T.; Egawa, Y.; Adachi, T.; Oguri, N.; Kobayashi, M.; Kudo, T.; Takeda, N.; Unno, M.; Tanaka, R. Synthesis, Structures, and Thermal Properties of Symmetric and Janus “Lantern Cage” Siloxanes. Chem. A Eur. J. 2019, 25, 1683–1686. [Google Scholar] [CrossRef]
- Liu, Y.; Onodera, K.; Takeda, N.; Ouali, A.; Unno, M. Synthesis and Characterization of Functionalizable Silsesquioxanes with Ladder-type Structures. Organometallics 2019, 38, 4373–4376. [Google Scholar] [CrossRef]
- Chaiprasert, T.; Liu, Y.; Takeda, N.; Unno, M. Janus ring siloxane: A versatile precursor of the extended Janus ring and tricyclic laddersiloxanes. Dalton Trans. 2020, 49, 13533–13537. [Google Scholar] [CrossRef]
- Asuncion, M.Z.; Ronchi, M.; Abu-Seir, H.; Laine, R.M. Synthesis, functionalization and properties of incompletely condensed “half cube” silsesquioxanes as a potential route to nanoscale Janus particles. Comptes Rendus Chim. 2010, 13, 270–281. [Google Scholar] [CrossRef]
- Oguri, N.; Egawa, Y.; Takeda, N.; Unno, M. Janus-Cube Octasilsesquioxane: Facile Synthesis and Structure Elucidation. An-gewandte Chem. Int. Ed. 2016, 55, 9336–9339. [Google Scholar] [CrossRef]
- Tateyama, S.; Kakihana, Y.; Kawakami, Y. Cage octaphenylsilsesquioxane from cyclic tetrasiloxanetetraol and its sodium salt. J. Organomet. Chem. 2010, 695, 898–902. [Google Scholar] [CrossRef]
- Inoue, H.; Matsukawa, K. Synthesis and Gas Permeability of Cyclotetrasiloxane-Containing Methacrylate Copolymers. J. Macromol. Sci. Part A 1992, 29, 415–440. [Google Scholar] [CrossRef]
- Gretton, M.J.; Kamino, B.A.; Bender, T.P. Extension of the Application of Piers-Rubinsztajn Conditions to Produce Tri-arylamine Pendant Dimethylsiloxane Copolymers. Macromol. Symp. 2013, 324, 82–94. [Google Scholar] [CrossRef]
- Wu, C.; Yu, J.; Li, Q.; Liu, Y. High molecular weight cyclic polysiloxanes from organocatalytic zwitterionic polymerization of constrained spirocyclosiloxanes. Polym. Chem. 2017, 8, 7301–7306. [Google Scholar] [CrossRef]
- Frampton, M.B.; Marquardt, D.; Jones, T.R.B.; Harroun, T.A.; Zelisko, P.M. Macrocyclic Oligoesters Incorporating a Cy-clotetrasiloxane Ring. Biomacromolecules 2015, 16, 2091–2100. [Google Scholar] [CrossRef]
- Casado, C.M.; Cuadrado, I.; Morán, M.; Alonso, B.; Barranco, M.; Losada, J. Cyclic siloxanes and silsesquioxanes as cores and frameworks for the construction of ferrocenyl dendrimers and polymers. Appl. Organomet. Chem. 1999, 13, 245–259. [Google Scholar] [CrossRef]
- Du, Y.; Unno, M.; Liu, H. Hybrid Nanoporous Materials Derived from Ladder- and Cage-Type Silsesquioxanes for Water Treatment. ACS Appl. Nano Mater. 2020, 3, 1535–1541. [Google Scholar] [CrossRef]
- Kamino, B.A.; Bender, T.P. The use of siloxanes, silsesquioxanes, and silicones in organic semiconducting materials. Chem. Soc. Rev. 2013, 42, 5119–5130. [Google Scholar] [CrossRef] [PubMed]
- Rodošek, M.; Koželj, M.; Slemenik Perše, L.; Cerc Korošec, R.; Gaberšček, M.; Surca, A.K. Protective coatings for AA 2024 based on cyclotetrasiloxane and various alkoxysilanes. Corros. Sci. 2017, 126, 55–68. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Kulakova, A.N.; Levitsky, M.M.; Petrov, A.A.; Korlyukov, A.A.; Shul’Pina, L.S.; Khrustalev, V.N.; Dorovatovskii, P.V.; Vologzhanina, A.V.; Tsareva, U.S.; et al. Unusual Tri-, Hexa-, and Nonanuclear Cu(II) Cage Methylsilsesquioxanes: Synthesis, Structures, and Catalytic Activity in Oxidations with Peroxides. Inorg. Chem. 2017, 56, 4093–4103. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Lamaty, F.; Bantreil, X.; Villemejeanne, B.; et al. Si10Cu6N4 Cage Hexacoppersilsesquioxanes Containing N Ligands: Synthesis, Structure, and High Catalytic Activity in Peroxide Oxidations. Inorg. Chem. 2017, 56, 15026–15040. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’Pina, L.S.; Kulakova, A.N.; Bantreil, X.; Lamaty, F.; Levitsky, M.M.; Gutsul, E.I.; Shubina, E.S.; et al. Heptanuclear Fe5Cu2-Phenylgermsesquioxane containing 2,2′-Bipyridine: Synthesis, Structure, and Catalytic Activity in Oxidation of C–H Compounds. Inorg. Chem. 2017, 57, 528–534. [Google Scholar] [CrossRef]
- Brown, J.F.; Vogt, L.H. The Polycondensation of Cyclohexylsilanetriol. J. Am. Chem. Soc. 1965, 87, 4313–4317. [Google Scholar] [CrossRef]
- Brown, J.F. The Polycondensation of Phenylsilanetriol. J. Am. Chem. Soc. 1965, 87, 4317–4324. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Anisimov, A.A.; Milenin, S.A.; Korlyukov, A.A.; Dolgushin, F.M.; Kononova, E.G.; Peregudov, A.S.; Buzin, M.I.; Shchegolikhina, O.I.; Muzafarov, A.M. New all-cis-tetra(p-tolyl)cyclotetrasiloxanetetraol and its functionaliza-tion. Mendeleev Commun. 2018, 28, 418–420. [Google Scholar] [CrossRef]
- Molodtsova, Y.; Pozdniakova, Y.; Lyssenko, K.; Blagodatskikh, I.; Katsoulis, D.; Shchegolikhina, O. A new approach to the synthesis of cage-like metallasiloxanes. J. Organomet. Chem. 1998, 571, 31–36. [Google Scholar] [CrossRef]
- Molodtsova, Y.A.; Shchegolikhina, O.I.; Peregudov, A.S.; Buzin, M.I.; Matukhina, E.V. Synthesis and mesomorphic prop-erties of cis-penta[(phenyl)(trimethylsiloxy)]cyclopentasiloxane. Russ. Chem. Bull. 2007, 56, 1402–1407. [Google Scholar] [CrossRef]
- Shchegolikhina, O.; Pozdniakova, Y.; Antipin, M.; Katsoulis, D.; Auner, N.; Herrschaft, B. Synthesis and Structure of Sodium Phenylsiloxanolate. Organometallics 2000, 19, 1077–1082. [Google Scholar] [CrossRef]
- Matukhina, E.V.; Shchegolikhina, O.I.; Makarova, N.N.; Pozdniakova, Y.A.; Katsoulis, D.E.; Godovsky, Y.K. New mesomorphic organocyclosiloxanes I. Thermal behaviour and mesophase structure of organocyclotetrasiloxanes. Liq. Cryst. 2001, 28, 869–879. [Google Scholar] [CrossRef]
- Pozdniakova, Y.A.; Lyssenko, K.A.; Korlyukov, A.A.; Blagodatskikh, I.V.; Auner, N.; Katsoulis, D.; Shchegolikhina, O.I. Alkali-Metal-Directed Hydrolytic Condensation of Trifunctional Phenylalkoxysilanes. Eur. J. Inorg. Chem. 2004, 2004, 1253–1261. [Google Scholar] [CrossRef]
- Shchegolikhina, O.I.; Pozdnyakova, Y.A.; Chetverikov, A.A.; Peregudov, A.S.; Buzin, M.I.; Matukhina, E.V. cis-Tetra[(organo)(trimethylsiloxy)]cyclotetrasiloxanes: Synthesis and mesomorphic properties. Russ. Chem. Bull. 2007, 56, 83–90. [Google Scholar] [CrossRef]
- Ronchi, M.; Pizzotti, M.; Biroli, A.O.; Macchi, P.; Lucenti, E.; Zucchi, C. Synthesis and structural characterization of functionalized cyclotetrasiloxane rings [4-RC6H4Si(O)OR′]4 (R = Cl, Br, CHCH2, CH2Cl; R′ = Na, SiMe3) as scaffolds for the synthesis of models of a silica bound monolayer of fluorescent or second order NLO active organic chromophores. J. Organomet. Chem. 2007, 692, 1788–1798. [Google Scholar] [CrossRef]
- Makarova, N.N.; Petrova, I.M.; Petrovskii, P.V.; Kaznacheev, A.V.; Volkova, L.M.; Shcherbina, M.A.; Bessonova, N.P.; Chvalun, S.N.; Godovskii, Y.K. Synthesis of new stereoregular 2,4,6,8-tetraphenylcyclotetrasiloxanes with mesogenic groups and the influence of spatial isomerism on the phase state of individual isomers and their mixtures. Russ. Chem. Bull. 2004, 53, 1983–1992. [Google Scholar] [CrossRef]
- Marciniec, B.; Waehner, J.; Pawluc, P.; Kubicki, M. Highly stereoselective synthesis and application of functionalized tetra-vinylcyclotetrasiloxanes via catalytic reactions. J. Mol. Catal. A Chem. 2007, 265, 25–31. [Google Scholar] [CrossRef]
- Kamino, B.A.; Mills, B.; Reali, C.; Gretton, M.J.; Brook, M.A.; Bender, T.P. Liquid Triarylamines: The Scope and Limitations of Piers–Rubinsztajn Conditions for Obtaining Triarylamine–Siloxane Hybrid Materials. J. Org. Chem. 2012, 77, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.A.; Grande, J.B.; Ganachaud, F. New Synthetic Strategies for Structured Silicones Using B(C6F5). In Silicon Polymers; Muzafarov, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 161–183. [Google Scholar]
- Brook, M.A. New Control Over Silicone Synthesis using SiH Chemistry: The Piers–Rubinsztajn Reaction. Chem. A Eur. J. 2018, 24, 8458–8469. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Shiba, H.; Wada, H.; Shimojima, A.; Kuroda, K. Polymerization of Cyclododecasiloxanes with Si–H and Si–OEt Side Groups by the Piers-Rubinsztajn Reaction. Bull. Chem. Soc. Jpn. 2018, 91, 747–753. [Google Scholar] [CrossRef]
- Kamino, B.A.; Grande, J.B.; Brook, M.A.; Bender, T.P. Siloxane−Triarylamine Hybrids: Discrete Room Temperature Liquid Triarylamines via the Piers−Rubinsztajn Reaction. Org. Lett. 2011, 13, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Shinke, S.; Tsuchimoto, T.; Kawakami, Y. Stereochemistry in Lewis acid-catalyzed silylation of alcohols, silanols, and meth-oxysilanes with optically active methyl(1-naphthyl)phenylsilane. Silicon Chem. 2007, 3, 243–249. [Google Scholar] [CrossRef]
- Grande, J.B.; Thompson, D.B.; Gonzaga, F.; Brook, M.A. Testing the functional tolerance of the Piers–Rubinsztajn reaction: A new strategy for functional silicones. Chem. Commun. 2010, 46, 4988–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.; Chen, T.; Hayes, R.; Dickie, T.; Urlich, T.; Brook, M.A. Facile synthesis of dendron-branched silicone polymers. Polym. Chem. 2017, 8, 2743–2746. [Google Scholar] [CrossRef]
- Schneider, A.F.; Chen, Y.; Brook, M.A. Trace water affects tris(pentafluorophenyl)borane catalytic activity in the Piers–Rubinsztajn reaction. Dalton Trans. 2019, 48, 13599–13606. [Google Scholar] [CrossRef]
- Chojnowski, J.; Rubinsztajn, S.; Cella, J.A.; Fortuniak, W.; Cypryk, M.; Kurjata, J.; Kaźmierski, K. Mechanism of the B(C6F5)3-Catalyzed Reaction of Silyl Hydrides with Alkoxysilanes. Kinetic and Spectroscopic Studies. Organometallics 2005, 24, 6077–6084. [Google Scholar] [CrossRef]
- Chojnowski, J.; Fortuniak, W.; Kurjata, J.; Rubinsztajn, S.; Cella, J.A. Oligomerization of Hydrosiloxanes in the Presence of Tris(pentafluorophenyl)borane. Macromolecules 2006, 39, 3802–3807. [Google Scholar] [CrossRef]
- Chojnowski, J.; Rubinsztajn, S.; Fortuniak, W.; Kurjata, J. Oligomer and Polymer Formation in Hexamethylcyclotrisiloxane (D3)–Hydrosilane Systems Under Catalysis by tris(pentafluorophenyl)borane. J. Inorg. Organomet. Polym. Mater. 2007, 17, 173–187. [Google Scholar] [CrossRef]
- Chojnowski, J.; Kurjata, J.; Fortuniak, W.; Rubinsztajn, S.; Trzebicka, B. Hydride Transfer Ring-Opening Polymerization of a Cyclic Oligomethylhydrosiloxane. Route to a Polymer of Closed Multicyclic Structure. Macromolecules 2012, 45, 2654–2661. [Google Scholar] [CrossRef]
- Zhou, D.; Kawakami, Y. Tris(pentafluorophenyl)borane as a Superior Catalyst in the Synthesis of Optically Active SiO-Containing Polymers. Macromolecules 2005, 38, 6902–6908. [Google Scholar] [CrossRef]
- Kawakami, Y.; Li, Y.; Liu, Y.; Seino, M.; Pakjamsai, C.; Oishi, M.; Cho, Y.H.; Imae, I. Control of molecular weight, stereo-chemistry and higher order structure of siloxane-containing polymers and their functional design. Macromol. Res. 2004, 12, 156–171. [Google Scholar] [CrossRef]
- Hoque, A.; Kakihana, Y.; Shinke, S.; Kawakami, Y. Polysiloxanes with Periodically Distributed Isomeric Double-Decker Silsesquioxane in the Main Chain. Macromolecules 2009, 42, 3309–3315. [Google Scholar] [CrossRef]
- Sodkhomkhum, R.; Ervithayasuporn, V. Synthesis of poly(siloxane/double-decker silsesquioxane) via dehydrocarbonative condensation reaction and its functionalization. Polymers 2016, 86, 113–119. [Google Scholar] [CrossRef]
- Rubinsztajn, S.; Cella, J.A. A New Polycondensation Process for the Preparation of Polysiloxane Copolymers. Macromolecules 2005, 38, 1061–1063. [Google Scholar] [CrossRef]
- Cella, J.; Rubinsztajn, S. Preparation of Polyaryloxysilanes and Polyaryloxysiloxanes by B(C6F5)3 Catalyzed Polyetherification of Dihydrosilanes and Bis-Phenols. Macromolecules 2008, 41, 6965–6971. [Google Scholar] [CrossRef]
- Blackwell, J.M.; Foster, K.L.; Beck, V.H.; Piers, W.E. B(C6F5)3-Catalyzed Silation of Alcohols: A Mild, General Method for Synthesis of Silyl Ethers. J. Org. Chem. 1999, 64, 4887–4892. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Lewandowski, D.; Hreczycho, G. B(C6F5)3-Catalyzed Dehydrocoupling of POSS Silanols with Hydrosilanes: A Metal-Free Strategy for Effecting Functionalization of Silsesquioxanes. Inorg. Chem. 2020, 59, 9206–9214. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oba, Y.; Nakajima, Y.; Shimada, S.; Sato, K. One-Pot Sequence-Controlled Synthesis of Oligosiloxanes. Angewandte Chem. Int. Ed. 2018, 57, 4637–4641. [Google Scholar] [CrossRef]
- Yang, W.; Gao, L.; Lu, J.; Song, Z.; Ji, L. Chemoselective deoxygenation of ether-substituted alcohols and carbonyl compounds by B(C6F5)3-catalyzed reduction with (HMe2SiCH2). Chem. Commun. 2018, 54, 4834–4837. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasert, T.; Liu, Y.; Intaraprecha, P.-k.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of Tricyclic Laddersiloxane with Various Ring Sizes (Bat Siloxane). Macromol. Rapid Commun. 2021, 42, 2000608. [Google Scholar] [CrossRef]
- Unno, M.; Suto, A.; Matsumoto, H. Pentacyclic Laddersiloxane. J. Am. Chem. Soc. 2002, 124, 1574–1575. [Google Scholar] [CrossRef]
- Chang, S.; Matsumoto, T.; Matsumoto, H.; Unno, M. Synthesis and characterization of heptacyclic laddersiloxanes and ladder polysilsesquioxane. Appl. Organomet. Chem. 2010, 24, 241–246. [Google Scholar] [CrossRef]
- Unno, M.; Matsumoto, T.; Matsumoto, H. Nonacyclic Ladder Silsesquioxanes and Spectral Features of Ladder Polysilsesqui-oxanes. Int. J. Polym. Sci. 2012, 2012, 723892. [Google Scholar] [CrossRef] [Green Version]
- Unno, M.; Suto, A.; Matsumoto, T. Laddersiloxanes—Silsesquioxanes with defined ladder structure. Russ. Chem. Rev. 2013, 82, 289–302. [Google Scholar] [CrossRef]
- Kunthom, R.; Adachi, T.; Liu, Y.; Takeda, N.; Unno, M.; Tanaka, R. Synthesis of a “Butterfly Cage” Based on a Double-Decker Silsesquioxane. Chem. Asian J. 2019, 14, 4179–4182. [Google Scholar] [CrossRef]
- Kunthom, R.; Takeda, N.; Unno, M. Synthesis and Characterization of Unsymmetrical Double-Decker Siloxane (Basket Cage). Molecules 2019, 24, 4252. [Google Scholar] [CrossRef] [PubMed] [Green Version]






 | |||
|---|---|---|---|
| Entry | Janus Rings | R | Isolated Yield 1 (%) |
| 1 | Vi-JR-01 |  | 60 |
| 2 | Vi-JR-02 |  | 55 |
| 3 | Vi-JR-03 |  | 42 |
| 4 | Vi-JR-04 |  | 46 |
| 5 | Vi-JR-05 |  | 39 |
| 6 | Vi-JR-06 |  | 38 |
| 7 | Vi-JR-07 |  | 50 |
| 8 | Vi-JR-08 |  | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiprasert, T.; Liu, Y.; Takeda, N.; Unno, M. Vinyl-Functionalized Janus Ring Siloxane: Potential Precursors to Hybrid Functional Materials. Materials 2021, 14, 2014. https://doi.org/10.3390/ma14082014
Chaiprasert T, Liu Y, Takeda N, Unno M. Vinyl-Functionalized Janus Ring Siloxane: Potential Precursors to Hybrid Functional Materials. Materials. 2021; 14(8):2014. https://doi.org/10.3390/ma14082014
Chicago/Turabian StyleChaiprasert, Thanawat, Yujia Liu, Nobuhiro Takeda, and Masafumi Unno. 2021. "Vinyl-Functionalized Janus Ring Siloxane: Potential Precursors to Hybrid Functional Materials" Materials 14, no. 8: 2014. https://doi.org/10.3390/ma14082014
APA StyleChaiprasert, T., Liu, Y., Takeda, N., & Unno, M. (2021). Vinyl-Functionalized Janus Ring Siloxane: Potential Precursors to Hybrid Functional Materials. Materials, 14(8), 2014. https://doi.org/10.3390/ma14082014