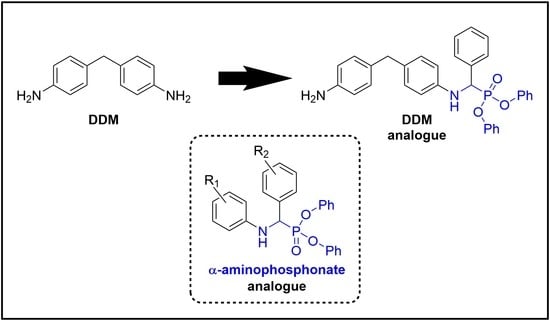
α-Aminophosphonate Derivatives for Enhanced Flame Retardant Properties in Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Sample Preparation
2.3. Analytical Techniques
2.3.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.3.2. Infrared Spectroscopy
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Limiting Oxygen Index
2.3.5. SEM
3. Results and Discussion
3.1. Synthesis and Characterisation
Chemical Structure of the Cured Network
3.2. Thermogravimetric Analysis and Flammability
3.3. Morphology of the Residue
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouritz, A.P.; Feih, S.; Kandare, E.; Mathys, Z.; Gibson, A.G.; Des Jardin, P.E.; Case, S.W.; Lattimer, B.Y. Review of fire structural modelling of polymer composites. Compos. Part A 2009, 40, 1800–1814. [Google Scholar] [CrossRef]
- Kandare, E.; Griffin, G.J.; Feih, S.; Gibson, A.G.; Lattimer, B.Y.; Mouritz, A.P. Fire structural modelling of fibre–polymer laminates protected with an intumescent coating. Compos. Part A 2012, 43, 793–802. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.-Z. A review on flame retardant technology in China. Part I: Development of flame retardants. Polym. Avd. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Agnieszka, B.; Paweł, K.; Bartłomiej, B.; Bogusław, T.; Jolanta, I.; Joanna, L. The application of organophosphorus flame-retardants in epoxy resin. J. Vinyl Addit. Technol. 2017, 23, 142–151. [Google Scholar]
- Wang, J.; Ma, C.; Wang, P.; Qiu, S.; Cai, W.; Hu, Y. Ultra-low phosphorus loading to achieve the superior flame retardancy of epoxy resin. Polym. Degrad. Stab. 2018, 149, 119–128. [Google Scholar] [CrossRef]
- Zhou, R.; Li, W.; Mu, J.; Ding, Y.; Jiang, J. Synergistic Effects of Aluminum Diethylphosphinate and Melamine on Improving the Flame Retardancy of Phenolic Resin. Materials 2020, 13, 158. [Google Scholar] [CrossRef] [Green Version]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Sonnier, R.; Otazaghine, B.; Vagner, C.; Bier, F.; Six, J.-L.; Durand, A.; Vahabi, H. Exploring the Contribution of Two Phosphorus-Based Groups to Polymer Flammability via Pyrolysis–Combustion Flow Calorimetry. Materials 2019, 12, 2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Luo, Q.; Yuan, Y.; Dong, C.; Liu, S.; Zhao, J. Intumescent flame retardancy of a DGEBA epoxy resin based on 5,10-dihydro-phenophosphazine-10-oxide. RSC Adv. 2015, 5, 68476–68484. [Google Scholar] [CrossRef]
- Chen, T.; Peng, C.; Liu, C.; Yuan, C.; Hong, J.; Chen, G.; Xu, Y.; Dai, L. Modification of Epoxy Resin with a Phosphorus, Nitrogen, and Fluorine Containing Polymer to Improve the Flame Retardant and Hydrophobic Properties. Macromol. Mater. Eng. 2019, 304, 1800498. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.; Qiu, Y.; Dong, Y. Synergistic flame-retardant effect and mechanisms of boron/phosphorus compounds on epoxy resins. Polym. Adv. Technol. 2018, 29, 641–648. [Google Scholar] [CrossRef]
- You, G.; Cheng, Z.; Peng, H.; He, H. The Synthesis and Characterization of a Novel Phosphorus–Nitrogen Containing Flame Retardant and its Application in Epoxy Resins. J. Appl. Polym Sci. 2014, 131, 41079. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. The impact of sulfur oxidation level on flame retardancy. J. Fire Sci. 2018, 36, 518–534. [Google Scholar] [CrossRef]
- Spontón, M.; Mercado, L.A.; Ronda, J.C.; Galià, M.; Cádiz, V. Preparation, thermal properties and flame retardancy of phosphorus—and silicon-containing epoxy resins. Polym. Degrad. Stab. 2008, 93, 2025–2031. [Google Scholar] [CrossRef]
- Na, T.; Jiang, H.; Zhao, L.; Zhao, C. Preparation and characterization of novel naphthyl epoxy resin containing 4-fluorobenzoyl side chains for low-k dielectrics application. RSC Adv. 2017, 7, 53970–53976. [Google Scholar] [CrossRef] [Green Version]
- Enescu, D.; Frache, A.; Lavaselli, M.; Monticelli, O.; Marino, F. Novel phosphorous–nitrogen intumescent flame retardant system. Its effects on flame retardancy and thermal properties of polypropylene. Polym. Degrad. Stab. 2013, 98, 297–305. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Hatanaka, L.C.; Mannan, M.S. Application of polymer nanocomposites in the flame retardancy study. J. Loss Prev. Process Ind. 2018, 55, 381–391. [Google Scholar] [CrossRef]
- Nazaré, S.; Kandola, B.K.; Horrocks, A.R. Flame-retardant unsaturated polyester resin incorporating nanoclays. Polym. Adv. Technol. 2006, 17, 294–303. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Zheng, X. Research on highly flame-retardant rigid PU foams by combination of nanostructured additives and phosphorus flame retardants. Polym. Degrad. Stab. 2015, 111, 142–150. [Google Scholar] [CrossRef]
- Toldya, A.; Szolnokib, B.; Marosi, G. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications. Polym. Degrad. Stab. 2011, 3, 371–376. [Google Scholar] [CrossRef]
- Jiana, R.; Wanga, P.; Xiaa, L.; Zheng, X. Effect of a novel P/N/S-containing reactive flame retardant on curing behavior, thermal and flame-retardant properties of epoxy resin. J. Anal. Appl. Pyrolysis 2017, 127, 360–368. [Google Scholar] [CrossRef]
- Stanfield, M.K.; Stojcevski, F.; Hendlmeier, A.; Varley, R.J.; Carrascal, J.; Osorio, A.F.; Eyckens, D.J.; Henderson, L.C. Phosphorus-Based α-Amino Acid Mimetic for Enhanced Flame-Retardant Properties in an Epoxy Resin. Aust. J. Chem. 2018, 72, 226–232. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Henderson, L.C. Synthesis of α-aminophosphonates using solvate ionic liquids. RSC Adv. 2017, 7, 27900–27904. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. D2863-19 Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index); ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wang, Y.Z.; Chang, F.C. Thermal and Flame Retardation Properties of Melamine Phosphate-Modified Epoxy Resins. J. Polym. Res. 2004, 11, 109–117. [Google Scholar] [CrossRef] [Green Version]








| Compound | Resin (g) | Hardener (g) | Compound (g) | P % |
|---|---|---|---|---|
| Control | 1.8 | 1.2 | 0 | 0 |
| 5–10 | 1.8 | 0.96 | 0.24 | ~0.60 |
| 11 | 1.8 | 0.96 | 0.24 | 0.48 |
| Compound | Char Yield (%) a | Char Yield (%) b | LOI (%) |
|---|---|---|---|
| Control | 35.8 | 21.1 | 28.0 ± 0.3 |
| 5 | 38.5 | 29.6 | 34.2 ± 0.2 |
| 6 | 33.8 | 33.2 | 32.8 ± 0.3 |
| 7 | 33.7 | 28.1 | 32.2 ± 0.2 |
| 8 | 35.4 | 31.6 | 34.6 ± 0.3 |
| 9 | 33.5 | 30.8 | 30.0 ± 0.1 |
| 10 | 33.1 | 32.0 | 33.0 ± 0.4 |
| 11 | 36.0 | 31.0 | 33.2 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanfield, M.K.; Carrascal, J.; Henderson, L.C.; Eyckens, D.J. α-Aminophosphonate Derivatives for Enhanced Flame Retardant Properties in Epoxy Resin. Materials 2021, 14, 3230. https://doi.org/10.3390/ma14123230
Stanfield MK, Carrascal J, Henderson LC, Eyckens DJ. α-Aminophosphonate Derivatives for Enhanced Flame Retardant Properties in Epoxy Resin. Materials. 2021; 14(12):3230. https://doi.org/10.3390/ma14123230
Chicago/Turabian StyleStanfield, Melissa K., Jeronimo Carrascal, Luke C. Henderson, and Daniel J. Eyckens. 2021. "α-Aminophosphonate Derivatives for Enhanced Flame Retardant Properties in Epoxy Resin" Materials 14, no. 12: 3230. https://doi.org/10.3390/ma14123230
APA StyleStanfield, M. K., Carrascal, J., Henderson, L. C., & Eyckens, D. J. (2021). α-Aminophosphonate Derivatives for Enhanced Flame Retardant Properties in Epoxy Resin. Materials, 14(12), 3230. https://doi.org/10.3390/ma14123230