Synthesis, Mesomorphism and the Optical Properties of Alkyl-deuterated Nematogenic 4-[(2,6-Difluorophenyl)ethynyl]biphenyls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Mesomrphic Properties and Discussion
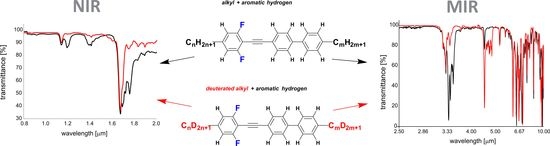
2.4. Optical Properties and Discussion
2.4.1. Medium Wavelength Infrared MWIR Absorption (IR-C)—2500–17,000 nm (4000–600 cm−1) Range
2.4.2. Near-Infrared NIR Absorption (IR-A + IR-B)—800–2000 nm (12,500–5000 cm−1) Range
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, K.K.; Tripathi, P.K.; Misra, A.K.; Manohar, R. UV response on dielectric properties of nano nematic liquid crystal. Results Phys. 2018, 8, 1119–1123. [Google Scholar] [CrossRef]
- Li, Q.R.; Qiu, J.; Liu, H.G.; Chen, X. A luminescent lyotropic liquid crystal with UV irradiation induced photochromism. Soft Matter 2020, 16, 1170–1178. [Google Scholar] [CrossRef]
- Chen, R.; An, Z.; Wang, W.; Chen, X.; Chen, P. Improving UV stability of tolane-liquid crystals in photonic applications by the ortho fluorine substitution. Opt. Mater. Express 2016, 6, 97–105. [Google Scholar] [CrossRef]
- Pandey, K.K.; Dixit, A.C.; Khan, M.S.; Tripathi, P.K.; Misra, A.K.; Manohar, R. Effect of UV light irradiation on the dielectric behaviour of liquid crystal/nano composite. Mol. Cryst. Liq. Cryst. 2017, 656, 89–95. [Google Scholar] [CrossRef]
- Wu, P.-C.; Yang, S.-Y.; Lee, W. Recovery of UV-degraded electrical properties of nematic liquid crystals doped with TiO2 nanoparticles. J. Mol. Liq. 2016, 218, 150–155. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.-C.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675–4702. [Google Scholar] [CrossRef] [PubMed]
- Beeckman, J.; Neyts, K.; Vanbrabant, P. Liquid-crystal photonic applications. Opt. Eng. 2011, 50. [Google Scholar] [CrossRef] [Green Version]
- Harmata, P.; Herman, J. New-Generation Liquid Crystal Materials for Application in Infrared Region. Materials 2021, 14, 2616. [Google Scholar] [CrossRef]
- Harmata, P.; Herman, J.; Kula, P. Liquid crystals for IR: Part I—synthesis and properties of perfluoroalkyl or perfluoroalkoxy terminated oligophenyls. Liq. Cryst. 2020, 47, 2122–2143. [Google Scholar] [CrossRef]
- Harmata, P.; Herman, J.; Kula, P. Liquid crystals for IR: Part II synthesis and properties of perfluoroalkyl- or perfluoroalkoxy-terminated tolanes. Liq. Cryst. 2020, 47, 2144–2160. [Google Scholar] [CrossRef]
- Kula, P.; Czerwiński, M.; Herman, J.; Harmata, P. Liquid Crystals for IR: Part III—Bi- and multicomponent mixtures based on perfluoroalkyl or perfluoroalkoxy terminated oligophenyls and tolanes. Liq. Cryst. 2020, 47, 2161–2170. [Google Scholar] [CrossRef]
- Saito, M.; Hayashi, K. Integration of liquid crystal elements for creating an infrared Lyot filter. Opt. Express 2013, 21, 11984–11993. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, P.; Xia, W.; Wang, J.; Zhou, C.; Zhang, H. A low-loss and high birefringence fluoride photonic crystal fiber in near infrared band. Optik 2019, 185, 772–776. [Google Scholar] [CrossRef]
- Kula, P.; Bennis, N.; Marć, P.; Harmata, P.; Gacioch, K.; Morawiak, P.; Jaroszewicz, L.R. Perdeuterated liquid crystals for near infrared applications. Opt. Mater. 2016, 60, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.B.; Xing, J.; Wei, C.; Sha, J.Q.; Zhang, G.B.; Lv, G.Q.; Zhu, J.; Qiu, L.Z. Near-infrared light directed reflection in a cholesteric liquid crystal. Opt. Mater. Express 2017, 7, 4163–4170. [Google Scholar] [CrossRef]
- Nayak, R.A.; Veerabhadraswamy, B.N.; Shankar Rao, D.S.; Sudhakar, A.A.; Yelamaggad, C.V. Room-Temperature, Deep-Red/NIR-Emissive, C3-Symmetric (n,π-conjugated) Columnar Liquid Crystals: C3h-Tris(keto-hydrazone)s. ACS Omega 2021, 6, 3291–3306. [Google Scholar] [CrossRef]
- Hacker, E.; Lauth, H.; Reichel, F. Optically addressable liquid-crystal spatial light modulators for VIS to NIR light modulation. SPIE 1998, 3292, 13–24. [Google Scholar]
- Sun, C.; Lu, J. A Tunable NIR Filter with Sphere Phase Liquid Crystal. Crystals 2019, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Guo, L.-X.; Lin, B.-P.; Zhang, X.-Q.; Sun, Y.; Yang, H. Near-Infrared Responsive Liquid Crystalline Elastomers Containing Photothermal Conjugated Polymers. Macromolecules 2016, 49, 4023–4030. [Google Scholar] [CrossRef]
- Kang, B.; Woo, J.H.; Choi, E.; Lee, H.-H.; Kim, E.S.; Kim, J.; Hwang, T.-J.; Park, Y.-S.; Kim, D.H.; Wu, J.W. Optical switching of near infrared light transmission in metamaterial-liquid crystal cell structure. Opt. Express 2010, 18, 16492–16498. [Google Scholar] [CrossRef]
- Bortolozzo, U.; Residori, S.; Huignard, J.-P. Transmissive liquid crystal light-valve for near-infrared applications. Appl. Opt. 2013, 52, E73–E77. [Google Scholar] [CrossRef]
- Wu, S.T. Infrared Properties of Nematic Liquid-Crystals—An Overview. Opt. Eng. 1987, 26, 120–128. [Google Scholar] [CrossRef]
- Wu, S.-T.; Cox, R.J. Potential infrared liquid crystals. Liq. Cryst. 1989, 5, 1415–1424. [Google Scholar] [CrossRef]
- Wu, S.-T.; Wang, Q.-H.; Kempe, M.D.; Kornfield, J.A. Perdeuterated cyanobiphenyl liquid crystals for infrared applications. J. Appl. Phys. 2002, 92, 7146–7148. [Google Scholar] [CrossRef] [Green Version]
- Lipiński, K.; Lipiński, T.; Chruściel, J.; Suszko-purzycka, A.; Rykowski, A. Synthesis and Mesomorphic Properties of Deuterated 4-n-Pentylphenyl-4′-n-Alkoxythiobenzoates 7S5-d26 and 8S5-d28. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1994, 239, 87–93. [Google Scholar] [CrossRef]
- Kula, P.; Herman, J.; Harmata, P.; Czerwiński, M. NIR and MWIR transparent liquid crystals. In Proceedings of the 2014 39th International Conference on Infrared, Millimeter, and Terahertz waves (IRMMW-THz), Tucson, AZ, USA, 14–19 September 2014. [Google Scholar]
- Gray, G.W.; Mosley, A. The Synthesis of Deuteriated 4-n-Alkyl-4′-Cyanobiphenyls. Mol. Cryst. Liq. Cryst. 1978, 48, 233–242. [Google Scholar] [CrossRef]
- Dong, R.Y. Recent NMR Studies of Thermotropic Liquid Crystals. Annu. Rep. NMR Spectrosc. 2016, 87, 41–174. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, H.; Sun, J.; Kula, P.; Dabrowski, R.; Tripathi, S.; Twieg, R.; Wu, S.-T. Low absorption liquid crystals for mid-wave infrared applications. Opt. Express 2011, 19, 10843–10848. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; An, Z.; Li, J.; Chen, H.; Peng, F.; Wu, S.-T.; Wang, X.; Li, M. Low mid-infrared absorption tolane liquid crystals terminated by 2,2-difluorovinyloxyl: Synthesis, characterization and properties. J. Mater. Chem. C 2016, 4, 4939–4945. [Google Scholar] [CrossRef]
- Hu, M.; An, Z.; Li, J.; Mo, L.; Yang, Z.; Li, J.; Che, Z.; Yang, X. Tolane liquid crystals bearing fluorinated terminal group and their mid-wave infrared properties. Liq. Cryst. 2014, 41, 1696–1702. [Google Scholar] [CrossRef]
- Peng, F.; Lee, Y.-H.; Chen, H.; Li, Z.; Bostwick, A.E.; Twieg, R.J.; Wu, S.-T. Low absorption chlorinated liquid crystals for infrared applications. Opt. Mater. Express 2015, 5, 1281–1288. [Google Scholar] [CrossRef]
- Gu, D.; Wen, B.; Mahajan, M.; Taber, D.; Winker, B.; Guthals, D.; Campbell, B.; Sox, D. High power liquid crystal spatial light modulators. SPIE 2006, 9, 427. [Google Scholar]
- Schmid, A.; Papernov, S.; Zheng, W.; Marshall, K.; Gunderman, T.; Lee, J.-C.; Guardalben, M.; Jacobs, S.D. Liquid-Crystal Materials for High Peak-Power Laser Applications. Mol. Cryst. Liq. Cryst. 1991, 207, 33–42. [Google Scholar] [CrossRef]
- Zhuang, Z.; Suh, S.-W.; Patel, J.S. Polarization controller using nematic liquid crystals. Opt. Lett. 1999, 24, 694–696. [Google Scholar] [CrossRef]
- Aharon, O.; Abdulhalim, I. Liquid crystal wavelength-independent continuous polarization rotator. Opt. Eng. 2010, 49, 034002. [Google Scholar] [CrossRef]
- Tani, T.; Shribak, M.; Oldenbourg, R. Living Cells and Dynamic Molecules Observed with the Polarized Light Microscope: The Legacy of Shinya Inoué. Biol. Bull. 2016, 231, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike-Tani, M.; Tominaga, T.; Oldenbourg, R.; Tani, T. Instantaneous polarized light imaging reveals activity dependent structural changes of dendrites in mouse hippocampal slices. bioRxiv 2019, 523571. [Google Scholar] [CrossRef] [Green Version]
- Abdulhalim, I. Non-display bio-optic applications of liquid crystals. Liq. Cryst. Today 2011, 20, 44–60. [Google Scholar] [CrossRef] [Green Version]
- Sattar, S.; Lapray, P.; Foulonneau, A.; Bigué, L. Review of spectral and polarization imaging systems. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 6 April 2020. [Google Scholar]
- Abdlaty, R.; Sahli, S.; Hayward, J.; Fang, Q. Hyperspectral imaging: Comparison of acousto-optic and liquid crystal tunable filters. SPIE 2018, 10573, 105732P. [Google Scholar]
- Wang, W.; Li, C.; Tollner, E.W.; Rains, G.C.; Gitaitis, R.D. A liquid crystal tunable filter based shortwave infrared spectral imaging system: Design and integration. Comput. Electron. Agric. 2012, 80, 126–134. [Google Scholar] [CrossRef]
- Messaadi, A.; Sánchez-López, M.d.M.; García-Martínez, P.; Vargas, A.; Moreno, I. Optical system for measuring the spectral retardance function in an extended range. J. Eur. Opt. Soc.-Rapid Publ. 2016, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, L.; Pu, Y.; Pratavieira, S.; Budansky, Y.; Alfano, R. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J. Biomed. Opt. 2014, 19, 056004. [Google Scholar] [CrossRef]
- García-Martínez, P.; Moreno, I.; Sánchez-López, M.d.M.; Gomis, J.; Martínez, P.; Cofré, A. Programmable Supercontinuum Laser Spectrum Generator Based on a Liquid-Crystal on Silicon Spatial Light Modulator. Front. Phys. 2021, 9. [Google Scholar] [CrossRef]
- Moreno, I.; Carrión, J.V.; Martínez, J.L.; García-Martínez, P.; Sánchez-López, M.M.; Campos, J. Optical retarder system with programmable spectral retardance. Opt. Lett. 2014, 39, 5483–5486. [Google Scholar] [CrossRef]
- Lee, Y.U.; Kim, J.; Wu, J.W. Electro-optic switching in metamaterial by liquid crystal. Nano Converg. 2015, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Herman, J.; Harmata, P.; Strzeżysz, O.; Czerwiński, M.; Urban, S.; Kula, P. Synthesis and properties of chosen 4-butyl-phenyltolane derivatives—On the influence of core substitution on birefringence, mesomorphic and dielectric properties. J. Mol. Liq. 2018, 267, 511–519. [Google Scholar] [CrossRef]
- Kula, P.; Aptacy, A.; Herman, J.; Wojciak, W.; Urban, S. The synthesis and properties of fluoro-substituted analogues of 4-butyl-4 ‘-[(4-butylphenyl)ethynyl]biphenyls. Liq. Cryst. 2013, 40, 482–491. [Google Scholar] [CrossRef]
- Buchecker, R.; Marck, G.; Schadt, M. New Nematics Incorporating a 2,6-Difluorophenyl Acetylene Group. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1995, 260, 93–105. [Google Scholar] [CrossRef]
- Seils, F.; Schadt, M. Liquid Crystals with a Chlorovinyl Side Chain; Effect of Structural Variations on the Dielectric Anisotropy. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A. Mol. Cryst. Liq. Cryst. 1995, 260, 127–138. [Google Scholar] [CrossRef]
- Herman, J.; Kula, P. The synthesis of chiral fluorinated 4-alkyl-4′-[(4-alkylphenyl)ethynyl]biphenyls. Tetrahedron Lett. 2013, 54, 3621–3623. [Google Scholar] [CrossRef]






| Acronym | Phase Transition Temperatures [°C] (Enthalpy [kJ mol−1]) | Temperature Range of Nematic Phase [°C] |
|---|---|---|
| nm_D |  | |
| 22_D | Cr 107.2 (23.0) N 163.9 Iso | 56.7 |
| 23_D | Cr 68.1 (17.5) N 174.8 Iso | 106.7 |
| 32_D | Cr 73.2 (24.7) N 179.3 Iso | 106.1 |
| 33_D | Cr 78.0 (26.6) N 186.8 Iso | 108.8 |
| 25_D | Cr 72.0 (20.0) N 160.3 Iso | 88.3 |
| 35_D | Cr 85.6 (24.8) N 170.3 Iso | 84.7 |
| nm_H |  | |
| 22_H | Cr 108.9 (22.7) N 165.4 Iso | 56.5 |
| 23_H | Cr 70.2 (18.8) N 173.8 Iso | 103.6 |
| 32_H | Cr 74.7 (26.9) N 175.2 Iso | 100.5 |
| 33_H | Cr 78.3 (26.9) N 186.5 Iso | 108.2 |
| 25_H | Cr 73.6 (17.6) N 159.6 Iso | 86.0 |
| 35_H | Cr 87.8 (24.1) N 169.7 Iso | 81.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, J.; Harmata, P.; Czerwiński, M.; Strzeżysz, O.; Pytlarczyk, M.; Zając, M.; Kula, P. Synthesis, Mesomorphism and the Optical Properties of Alkyl-deuterated Nematogenic 4-[(2,6-Difluorophenyl)ethynyl]biphenyls. Materials 2021, 14, 4653. https://doi.org/10.3390/ma14164653
Herman J, Harmata P, Czerwiński M, Strzeżysz O, Pytlarczyk M, Zając M, Kula P. Synthesis, Mesomorphism and the Optical Properties of Alkyl-deuterated Nematogenic 4-[(2,6-Difluorophenyl)ethynyl]biphenyls. Materials. 2021; 14(16):4653. https://doi.org/10.3390/ma14164653
Chicago/Turabian StyleHerman, Jakub, Piotr Harmata, Michał Czerwiński, Olga Strzeżysz, Marta Pytlarczyk, Monika Zając, and Przemysław Kula. 2021. "Synthesis, Mesomorphism and the Optical Properties of Alkyl-deuterated Nematogenic 4-[(2,6-Difluorophenyl)ethynyl]biphenyls" Materials 14, no. 16: 4653. https://doi.org/10.3390/ma14164653
APA StyleHerman, J., Harmata, P., Czerwiński, M., Strzeżysz, O., Pytlarczyk, M., Zając, M., & Kula, P. (2021). Synthesis, Mesomorphism and the Optical Properties of Alkyl-deuterated Nematogenic 4-[(2,6-Difluorophenyl)ethynyl]biphenyls. Materials, 14(16), 4653. https://doi.org/10.3390/ma14164653