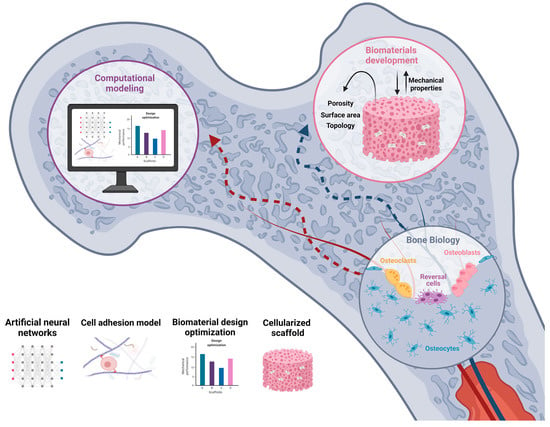
A Multidisciplinary Journey towards Bone Tissue Engineering
Abstract
:1. Introduction
2. Bone Biology
2.1. Bone Composition
2.1.1. Osteoblasts
2.1.2. Osteocytes
2.1.3. Osteoclasts
2.1.4. Bone-Lining Cells
2.1.5. Bone Matrix
2.2. Bone Remodeling Process
2.2.1. Activation
2.2.2. Resorption
2.2.3. Reversal
2.2.4. Formation
2.2.5. Termination

3. Bone Pathology
3.1. Osteoporosis
3.2. Paget’s Disease
3.3. Osteoarthritis
3.4. Autoinflammatory Diseases
3.5. Bone Metastasis
3.6. Therapeutics in Bone Disease
4. Scaffolds in Bone Tissue Engineering
4.1. Scaffold Composition
4.1.1. Polymeric Scaffolds
4.1.2. Bioceramics
4.1.3. Composite Materials
4.1.4. Nanomaterials
4.2. Scaffold Properties for BTE
| Surface area | Crucial for cell–scaffold interactions, facilitating vascularization and tissue infiltration | [54] |
| Macroporosity might promote osteogenesis by facilitating cell and ion transport | [58] | |
| Microporosity improves surface area for protein adsorption, increasing ionic solubility and attachment points for osteoblasts | [58] | |
| Pore size | Pores > 300 µm facilitate new bone formation and vascularization | [54] |
| 75–100 µm pore size is thought to promote angiogenesis | [6] | |
| Pore size range from 200 to 500 μm results in optimal tissue penetration vascularization in vivo | [6,21] | |
| Pore interconnectivity | Enhanced bone deposition rate and depth of infiltration | [58] |
| Optimal diameter of connections between pores ranges from 700–1200 µm | [54,59] | |
| Surface topology | Roughened surfaces promote osteointegration and favor epithelial attachment | [61] |
| Mechanical properties | Young’s modulus should be close to 7–30 GPa and a tensile strength of 50–151 MPa | [62] |
| Compressive strength should be comparable to cortical bone (100–230 MPa) | [62] | |
| Degradation rate should match the growth of native ECM to ensure scaffold mechanical support | [21,44] |
4.3. Scaffolds as Vehicles of Cells and Growth Factors
5. Cell–Biomaterial Interactions beyond Microenvironment
5.1. Cell Response to Biomaterial Chemistry
5.2. Cell Response to Biomaterial Topography
5.3. Cell Response to Biomaterial Elasticity
5.4. Cell Response to Mechanical Deformation
5.5. Organ-on-a-Chip 3D Culture
5.6. Extracellular Matrix and Cell–Biomaterial Interactions
5.7. Effect of Mechanical Forces on Cells and Tissues
5.8. Mechanical Forces in the Bone
6. Computational Modeling
6.1. Bone Mechanobiology
6.2. Cell Adhesion
6.3. Optimization of Scaffold Design
6.4. Machine Learning for 3D Printing
6.5. Computerized Multiscale Diagnostic System
7. Current Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bFGF | Basic Fibroblast Growth Factor |
| BMD | Bone Mineral Density |
| BMP | Bone Morphogenic Protein |
| BMU | Basic Multicellular Unit |
| BRC | Bone Remodeling Compartment |
| BTE | Bone Tissue Engineering |
| CAP | Cell Adhesion Protein |
| CPC | Calcium Phosphate Ceramic |
| CS | Chitosan |
| CSF-1 | Colony-stimulating Factor 1 |
| ECM | Extracellular Matrix |
| ESC | Embryonic Stem Cell |
| EPC | Endothelial Progenitor Cell |
| FAK | Focal Adhesion Kinase |
| FDA | Food and Drug Administration |
| GF | Growth Factor |
| GFOGER | Gly-Phe-Hyp-Gly-Glu-Arg |
| HA | Hydroxyapatite |
| hMSC | Human Mesenchymal Stem Cell |
| IL | Interleukin |
| iPSC | Induced Pluripotent Stem Cell |
| LINC | Linker of Nucleoskeleton and Cytoskeleton |
| MLaHA/CS | Magnetic Lanthanum (La)-doped HA Nanoparticles/Chitosan Scaffolds |
| MMP | Matrix Metalloproteinase |
| MP | Microparticle |
| MSC | Mesenchymal Stem Cell |
| MSN | Mesoporous Silica Nanoparticle |
| MWCNT | Multi-walled Carbon Nanotube |
| NP | Nanoparticle |
| OPG | Osteoprotegerin |
| OS | Osteosarcoma |
| PEEK | Polyether Ether Ketone |
| PCL | Poly(caprolactone) |
| PDGF | Platelet-derived Growth Factor |
| PEG | Polyethylene Glycol |
| PGA | Poly(glycolic acid) |
| PLA | Poly(lactic acid) |
| PLGA | Poly Lactic-co-Glycolic Acid |
| PMMA | Poly (methyl metacrylate) |
| PPF | Polypropylene Fumarate |
| PTH | Parathyroid Hormone |
| PTHrP | Parathyroid Hormone-related Peptide |
| RANKL | Receptor Activator of NF-κB Ligand |
| RGD | Arg-Gly-Asp |
| rMSC | Rat Mesenchymal Stem Cell |
| RUNX2 | Runt-related Transcription Factor 2 |
| SF | Silk Fibroin |
| Smurf1 | Smad Ubiquitin Regulatory Factor-1 |
| TCP | Tricalcium Phosphate |
| TNF | Tumoral Necrosis Factor |
| TGF-ꞵ | Transcription Growth Factor β |
| VEGF | Vascular Endothelial Growth Factor |
References
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Adv. Healthc. Mater. 2018, 7, 1701061. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Advancements and Frontiers in Nano-Based 3D and 4D Scaffolds for Bone and Cartilage Tissue Engineering. IJN 2019, 14, 4333–4351. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the Art and New Perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Ng, P.Y.; Ong, A.J.; Gale, L.S.; Dass, C.R. Treatment of Bone Disorders with Parathyroid Hormone: Success and Pitfalls. Pharmazie 2016, 71, 427–433. [Google Scholar]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current State of Fabrication Technologies and Materials for Bone Tissue Engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Vieira, S.; Vial, S.; Reis, R.L.; Oliveira, J.M. Nanoparticles for Bone Tissue Engineering. Biotechnol. Prog. 2017, 33, 590–611. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M. Bone Tissue Regeneration: Biology, Strategies and Interface Studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of Critical-Sized Bone Defects: Clinical and Tissue Engineering Perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Carlier, A.; Lammens, J.; Van Oosterwyck, H.; Geris, L. Computational Modeling of Bone Fracture Non-Unions: Four Clinically Relevant Case Studies. Silico. Cell Tissue Sci. 2015, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Buck, D.W.; Dumanian, G.A. Bone Biology and Physiology: Part I. The Fundamentals. Plast. Reconstr. Surg. 2012, 129, 7. [Google Scholar] [CrossRef]
- Sommerfeldt, D.W.; Rubin, C.T. Biology of Bone and How It Orchestrates the Form and Function of the Skeleton. Eur. Spine J. 2001, 10, S86–S95. [Google Scholar] [CrossRef] [Green Version]
- Katsimbri, P. The Biology of Normal Bone Remodelling. Eur. J. Cancer Care 2017, 26, e12740. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. In Frontiers of Oral Biology; Kantarci, A., Will, L., Yen, S., Eds.; S. Karger AG: Basel, Switzerland, 2015; Volume 18, pp. 9–16. ISBN 978-3-318-05479-8. [Google Scholar]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone Modeling and Remodeling: Potential as Therapeutic Targets for the Treatment of Osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, A.; Wolf, S.; Weiser, E.; Vater, C.; Gelinsky, M. An Improved Method to Isolate Primary Human Osteocytes from Bone. Biomed. Eng. Biomed. Tech. 2020, 65, 107–111. [Google Scholar] [CrossRef]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The Cell Biology of Bone Metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Brown, J.L.; Kumbar, S.G.; Laurencin, C.T. Bone Tissue Engineering. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1194–1214. ISBN 978-0-12-374626-9. [Google Scholar]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An Introduction to Bone Tissue Engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Matsumoto, M.A.; Biguetti, C.C.; Fonseca, A.C.; Saraiva, P.P. Bone Tissue Healing Dynamics: From Damage to Reconstruction. J. Mol. Signal. Updates 2016, 1, 33–40. [Google Scholar]
- Kenkre, J.S.; Bassett, J. The Bone Remodelling Cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Fuchs, R.K.; Warden, S.J.; Turner, C.H. Bone anatomy, physiology and adaptation to mechanical loading. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2009; pp. 25–68. ISBN 978-1-84569-385-5. [Google Scholar]
- Clarke, B. Normal Bone Anatomy and Physiology. CJASN 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Lu, L.; Yaszemski, M.J. Bone Disorders. In Materials for Bone Disorders; Elsevier: Amsterdam, The Netherlands, 2017; pp. 83–118. ISBN 978-0-12-802792-9. [Google Scholar]
- Jimi, E.; Hirata, S.; Osawa, K.; Terashita, M.; Kitamura, C.; Fukushima, H. The Current and Future Therapies of Bone Regeneration to Repair Bone Defects. Int. J. Dent. 2012, 2012, 148261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frohlich, M.; Grayson, W.; Wan, L.; Marolt, D.; Drobnic, M.; Vunjak- Novakovic, G. Tissue Engineered Bone Grafts: Biological Requirements, Tissue Culture and Clinical Relevance. CSCR 2008, 3, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Cawthray, J.; Wasan, E.; Wasan, K. Bone-Seeking Agents for the Treatment of Bone Disorders. Drug Deliv. Transl. Res. 2017, 7, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [Green Version]
- Griz, L.; Fontan, D.; Mesquita, P.; Lazaretti-Castro, M.; Borba, V.Z.C.; Borges, J.L.C.; Fontenele, T.; Maia, J.; Bandeira, F. Diagnosis and Management of Paget?S Disease of Bone. Arq. Bras. Endocrinol. Metab. 2014, 58, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Aerzteblatt. Online 2010, 107, 152. [Google Scholar] [CrossRef]
- Morbach, H.; Hedrich, C.M.; Beer, M.; Girschick, H.J. Autoinflammatory Bone Disorders. Clin. Immunol. 2013, 147, 185–196. [Google Scholar] [CrossRef]
- Stern, S.M.; Ferguson, P.J. Autoinflammatory Bone Diseases. Rheum. Dis. Clin. North. Am. 2013, 39, 735–749. [Google Scholar] [CrossRef] [Green Version]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Sun, W.; Ge, K.; Jin, Y.; Han, Y.; Zhang, H.; Zhou, G.; Yang, X.; Liu, D.; Liu, H.; Liang, X.-J.; et al. Bone-Targeted Nanoplatform Combining Zoledronate and Photothermal Therapy To Treat Breast Cancer Bone Metastasis. ACS Nano 2019, 13, 7556–7567. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Burg, K.J.L. Bone Tissue Engineering and Regenerative Medicine: Targeting Pathological Fractures: Bone Tissue Engineering and Regenerative Medicine. J. Biomed. Mater. Res. 2015, 103, 420–429. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Mao, J. Bone Tissue Engineering and Regeneration: From Discovery to the Clinic—An Overview. Tissue Eng. Part. B: Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.H.; Annabi, N.; Nikkhah, M.; Bae, H.; Binan, L.; Park, S.; Kang, Y.; Yang, Y.; Khademhosseini, A. Vascularized Bone Tissue Engineering: Approaches for Potential Improvement. Tissue Eng. Part. B: Rev. 2012, 18, 363–382. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic Approaches in Bone Tissue Engineering: Integrating Biological and Physicomechanical Strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Kalsi, S.; Singh, J.; Sehgal, S.S.; Sharma, N.K. Biomaterials for Tissue Engineered Bone Scaffolds: A Review. Mater. Today: Proc. 2021, in press. [Google Scholar]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions — A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisani, M.; Pezzoli, D.; Chassande, O.; Mantovani, D. Cellularizing Hydrogel-Based Scaffolds to Repair Bone Tissue: How to Create a Physiologically Relevant Micro-Environment? J. Tissue Eng. 2017, 8, 204173141771207. [Google Scholar] [CrossRef] [Green Version]
- Griffin, K.S.; Davis, K.M.; McKinley, T.O.; Anglen, J.O.; Chu, T.-M.G.; Boerckel, J.D.; Kacena, M.A. Evolution of Bone Grafting: Bone Grafts and Tissue Engineering Strategies for Vascularized Bone Regeneration. Clin. Rev. Bone Min. Metab. 2015, 13, 232–244. [Google Scholar] [CrossRef]
- Lamers, E.; te Riet, J.; Domanski, M.; Luttge, R.; Figdor, C.; Gardeniers, J.; Walboomers, X.; Jansen, J. Dynamic Cell Adhesion and Migration on Nanoscale Grooved Substrates. eCM 2012, 23, 182–194. [Google Scholar] [CrossRef]
- Stevens, M.M. Biomaterials for Bone Tissue Engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Yan, L.-P.; Silva-Correia, J.; Correia, C.; Caridade, S.G.; Fernandes, E.M.; Sousa, R.A.; Mano, J.F.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Bioactive Macro/Micro Porous Silk Fibroin/Nano-Sized Calcium Phosphate Scaffolds with Potential for Bone-Tissue-Engineering Applications. Nanomedicine 2013, 8, 359–378. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Cheng, Q.; Rutledge, K.; Jabbarzadeh, E. Carbon Nanotube–Poly(Lactide-Co-Glycolide) Composite Scaffolds for Bone Tissue Engineering Applications. Ann. Biomed. Eng 2013, 41, 904–916. [Google Scholar] [CrossRef]
- Cha, W.; Fan, R.; Miao, Y.; Zhou, Y.; Qin, C.; Shan, X.; Wan, X.; Li, J. Mesoporous Silica Nanoparticles as Carriers for Intracellular Delivery of Nucleic Acids and Subsequent Therapeutic Applications. Molecules 2017, 22, 782. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.; Perikamana, S.K.M.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthc. Mater. 2019, 8, 1801106. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The Mechanical Properties and Osteoconductivity of Hydroxyapatite Bone Scaffolds with Multi-Scale Porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Rubert, M.; Müller, R. 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors. IJMS 2021, 22, 3971. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Podshivalov, L.; Fischer, A.; Bar-Yoseph, P.Z. On the Road to Personalized Medicine: Multiscale Computational Modeling of Bone Tissue. Arch. Comput. Methods Eng 2014, 21, 399–479. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef]
- Bisht, B.; Hope, A.; Mukherjee, A.; Paul, M.K. Advances in the Fabrication of Scaffold and 3D Printing of Biomimetic Bone Graft. Ann. Biomed. Eng 2021, 49, 1128–1150. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Fathi, A.; Padashi, M.; Abu Osman, N.A. Optimal Design of a 3D-Printed Scaffold Using Intelligent Evolutionary Algorithms. Appl. Soft Comput. 2016, 39, 36–47. [Google Scholar] [CrossRef]
- Ni, P.; Ding, Q.; Fan, M.; Liao, J.; Qian, Z.; Luo, J.; Li, X.; Luo, F.; Yang, Z.; Wei, Y. Injectable Thermosensitive PEG–PCL–PEG Hydrogel/Acellular Bone Matrix Composite for Bone Regeneration in Cranial Defects. Biomaterials 2014, 35, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cao, S.; Chen, X.; Wu, W.; Li, J. Thermoresponsive Hydrogels from Phosphorylated ABA Triblock Copolymers: A Potential Scaffold for Bone Tissue Engineering. Biomacromolecules 2013, 14, 2206–2214. [Google Scholar] [CrossRef]
- Watson, B.M.; Kasper, F.K.; Engel, P.S.; Mikos, A.G. Synthesis and Characterization of Injectable, Biodegradable, Phosphate-Containing, Chemically Cross-Linkable, Thermoresponsive Macromers for Bone Tissue Engineering. Biomacromolecules 2014, 15, 1788–1796. [Google Scholar] [CrossRef]
- Marolt, D.; Campos, I.M.; Bhumiratana, S.; Koren, A.; Petridis, P.; Zhang, G.; Spitalnik, P.F.; Grayson, W.L.; Vunjak-Novakovic, G. Engineering Bone Tissue from Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 8705–8709. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Chen, W.; Weir, M.D.; Thein-Han, W.; Xu, H.H.K. Human Embryonic Stem Cell Encapsulation in Alginate Microbeads in Macroporous Calcium Phosphate Cement for Bone Tissue Engineering. Acta Biomater. 2012, 8, 3436–3445. [Google Scholar] [CrossRef] [Green Version]
- Bastami, F.; Nazeman, P.; Moslemi, H.; Rezai Rad, M.; Sharifi, K.; Khojasteh, A. Induced Pluripotent Stem Cells as a New Getaway for Bone Tissue Engineering: A Systematic Review. Cell Prolif. 2017, 50, e12321. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A. Applied Induced Pluripotent Stem Cells in Combination With Biomaterials in Bone Tissue Engineering. J. Cell. Biochem. 2017, 118, 3034–3042. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. IJMS 2021, 22, 1128. [Google Scholar] [CrossRef]
- Prosecká, E.; Rampichová, M.; Litvinec, A.; Tonar, Z.; Králíčková, M.; Vojtová, L.; Kochová, P.; Plencner, M.; Buzgo, M.; Míčková, A.; et al. Collagen/Hydroxyapatite Scaffold Enriched with Polycaprolactone Nanofibers, Thrombocyte-Rich Solution and Mesenchymal Stem Cells Promotes Regeneration in Large Bone Defect in Vivo: Coll/HA SCAFFOLD ENRICHED WITH PCL, MSCs AND TRS IN VIVO. J. Biomed. Mater. Res. 2015, 103, 671–682. [Google Scholar] [CrossRef]
- Checa, S.; Prendergast, P.J. Effect of Cell Seeding and Mechanical Loading on Vascularization and Tissue Formation inside a Scaffold: A Mechano-Biological Model Using a Lattice Approach to Simulate Cell Activity. J. Biomech. 2010, 43, 961–968. [Google Scholar] [CrossRef]
- Rodríguez-Évora, M.; García-Pizarro, E.; del Rosario, C.; Pérez-López, J.; Reyes, R.; Delgado, A.; Rodríguez-Rey, J.C.; Évora, C. Smurf1 Knocked-Down, Mesenchymal Stem Cells and BMP-2 in an Electrospun System for Bone Regeneration. Biomacromolecules 2014, 15, 1311–1322. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Furuya, H.; Tabata, Y. Enhancement of Bone Regeneration by Dual Release of a Macrophage Recruitment Agent and Platelet-Rich Plasma from Gelatin Hydrogels. Biomaterials 2014, 35, 214–224. [Google Scholar] [CrossRef] [Green Version]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone Tissue Engineering via Growth Factor Delivery: From Scaffolds to Complex Matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.; Bishop, T.; Valerio, I.L.; Fisher, J.P.; Dean, D. The Potential Impact of Bone Tissue Engineering in the Clinic. Regen. Med. 2016, 11, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Gaihre, B.; Bharadwaz, A.; Unagolla, J.M.; Jayasuriya, A.C. Evaluation of the Optimal Dosage of BMP-9 through the Comparison of Bone Regeneration Induced by BMP-9 versus BMP-2 Using an Injectable Microparticle Embedded Thermosensitive Polymeric Carrier in a Rat Cranial Defect Model. Mater. Sci. Eng. C 2021, 127, 112252. [Google Scholar] [CrossRef]
- Yu, L.; Dawson, L.A.; Yan, M.; Zimmel, K.; Lin, Y.-L.; Dolan, C.P.; Han, M.; Muneoka, K. BMP9 Stimulates Joint Regeneration at Digit Amputation Wounds in Mice. Nat. Commun. 2019, 10, 424. [Google Scholar] [CrossRef]
- Ferracini, R.; Martínez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as Structural Tools for Bone-Targeted Drug Delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- Chollet, C.; Chanseau, C.; Remy, M.; Guignandon, A.; Bareille, R.; Labrugère, C.; Bordenave, L.; Durrieu, M.-C. The Effect of RGD Density on Osteoblast and Endothelial Cell Behavior on RGD-Grafted Polyethylene Terephthalate Surfaces. Biomaterials 2009, 30, 711–720. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD Modified Polymers: Biomaterials for Stimulated Cell Adhesion and Beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Harbers, G.M.; Healy, K.E. The Effect of Ligand Type and Density on Osteoblast Adhesion, Proliferation, and Matrix Mineralization. J. Biomed. Mater. Res. 2005, 75, 855–869. [Google Scholar] [CrossRef]
- Kantlehner, M.; Schaffner, P.; Finsinger, D.; Meyer, J.; Jonczyk, A.; Diefenbach, B.; Nies, B.; Hölzemann, G.; Goodman, S.L.; Kessler, H. Surface Coating with Cyclic RGD Peptides Stimulates Osteoblast Adhesion and Proliferation as Well as Bone Formation. Chembiochem 2000, 1, 107–114. [Google Scholar] [CrossRef]
- Lieb, E.; Hacker, M.; Tessmar, J.; Kunz-Schughart, L.A.; Fiedler, J.; Dahmen, C.; Hersel, U.; Kessler, H.; Schulz, M.B.; Göpferich, A. Mediating Specific Cell Adhesion to Low-Adhesive Diblock Copolymers by Instant Modification with Cyclic RGD Peptides. Biomaterials 2005, 26, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone Regeneration Using an Alpha 2 Beta 1 Integrin-Specific Hydrogel as a BMP-2 Delivery Vehicle. Biomaterials 2014, 35, 5453–5461. [Google Scholar] [CrossRef] [Green Version]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscale Technologies for Tissue Engineering and Biology. Proc. Natl. Acad. Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khademhosseini, A.; Jon, S.; Suh, K.Y.; Tran, T.-N.T.; Eng, G.; Yeh, J.; Seong, J.; Langer, R. Direct Patterning of Protein- and Cell-Resistant Polymeric Monolayers and Microstructures. Adv. Mater. 2003, 15, 1995–2000. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Chiu, D.T.; Jeon, N.L.; Huang, S.; Kane, R.S.; Wargo, C.J.; Choi, I.S.; Ingber, D.E.; Whitesides, G.M. Patterned Deposition of Cells and Proteins onto Surfaces by Using Three-Dimensional Microfluidic Systems. Proc. Natl. Acad. Sci. USA 2000, 97, 2408–2413. [Google Scholar] [CrossRef] [Green Version]
- Takayama, S.; Ostuni, E.; LeDuc, P.; Naruse, K.; Ingber, D.E.; Whitesides, G.M. Subcellular Positioning of Small Molecules. Nature 2001, 411, 1016. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Micropatterned Surfaces for Control of Cell Shape, Position, and Function. Biotechnol. Prog. 1998, 14, 356–363. [Google Scholar] [CrossRef]
- Edahiro, J.; Sumaru, K.; Tada, Y.; Ohi, K.; Takagi, T.; Kameda, M.; Shinbo, T.; Kanamori, T.; Yoshimi, Y. In Situ Control of Cell Adhesion Using Photoresponsive Culture Surface. Biomacromolecules 2005, 6, 970–974. [Google Scholar] [CrossRef]
- Kikuchi, K.; Sumaru, K.; Edahiro, J.-I.; Ooshima, Y.; Sugiura, S.; Takagi, T.; Kanamori, T. Stepwise Assembly of Micropatterned Co-Cultures Using Photoresponsive Culture Surfaces and Its Application to Hepatic Tissue Arrays. Biotechnol. Bioeng. 2009, 103, 552–561. [Google Scholar] [CrossRef]
- Anderson, D.G.; Levenberg, S.; Langer, R. Nanoliter-Scale Synthesis of Arrayed Biomaterials and Application to Human Embryonic Stem Cells. Nat. Biotechnol. 2004, 22, 863–866. [Google Scholar] [CrossRef]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of Surface Topographical Cues on the Differentiation of Mesenchymal Stem Cells in Vitro. ACS Biomater. Sci. Eng. 2016, 2, 142–151. [Google Scholar] [CrossRef]
- Fratzl, P. When the Cracks Begin to Show. Nat. Mater. 2008, 7, 610–612. [Google Scholar] [CrossRef]
- Carinci, F.; Pezzetti, F.; Volinia, S.; Francioso, F.; Arcelli, D.; Marchesini, J.; Scapoli, L.; Piattelli, A. Analysis of Osteoblast-Like MG63 Cells’ Response to A Rough Implant Surface by means of DNA Microarray. J. Oral Implantol. 2003, 29, 215–220. [Google Scholar] [CrossRef]
- Hamilton, D.W.; Brunette, D.M. The Effect of Substratum Topography on Osteoblast Adhesion Mediated Signal Transduction and Phosphorylation. Biomaterials 2007, 28, 1806–1819. [Google Scholar] [CrossRef]
- Clark, P.; Connolly, P.; Curtis, A.S.G.; Dow, J.A.T.; Wilkinson, C.D.W. Topographical Control of Cell Behaviour. Development 1990, 108, 635–644. [Google Scholar] [CrossRef]
- Martínez, E.; Lagunas, A.; Mills, C.; Rodríguez-Seguí, S.; Estévez, M.; Oberhansl, S.; Comelles, J.; Samitier, J. Stem Cell Differentiation by Functionalized Micro- and Nanostructured Surfaces. Nanomedicine 2009, 4, 65–82. [Google Scholar] [CrossRef]
- Hacking, S.A.; Harvey, E.; Roughley, P.; Tanzer, M.; Bobyn, J. The Response of Mineralizing Culture Systems to Microtextured and Polished Titanium Surfaces. J. Orthop. Res. 2008, 26, 1347–1354. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Orrick, B.; Misra, A.; Langer, R.; Borenstein, J.T. Microfabrication of Poly (Glycerol–Sebacate) for Contact Guidance Applications. Biomaterials 2006, 27, 2558–2565. [Google Scholar] [CrossRef]
- Dalby, M.J.; Riehle, M.O.; Johnstone, H.; Affrossman, S.; Curtis, A.S.G. In Vitro Reaction of Endothelial Cells to Polymer Demixed Nanotopography. Biomaterials 2002, 23, 2945–2954. [Google Scholar] [CrossRef]
- Ball, M.D.; Prendergast, U.; O’Connell, C.; Sherlock, R. Comparison of Cell Interactions with Laser Machined Micron- and Nanoscale Features in Polymer. Exp. Mol. Pathol. 2007, 82, 130–134. [Google Scholar] [CrossRef]
- Curtis, A.S.G.; Gadegaard, N.; Dalby, M.J.; Riehle, M.O.; Wilkinson, C.D.W.; Aitchison, G. Cells React to Nanoscale Order and Symmetry in Their Surroundings. IEEE Trans. Nanobioscience 2004, 3, 61–65. [Google Scholar] [CrossRef]
- Kantawong, F.; Burchmore, R.; Gadegaard, N.; Oreffo, R.O.C.; Dalby, M.J. Proteomic Analysis of Human Osteoprogenitor Response to Disordered Nanotopography. J. R. Soc. Interface. 2009, 6, 1075–1086. [Google Scholar] [CrossRef] [Green Version]
- van Beuningen, H.M.; Glansbeek, H.L.; van der Kraan, P.M.; van den Berg, W.B. Osteoarthritis-like Changes in the Murine Knee Joint Resulting from Intra-Articular Transforming Growth Factor-β Injections. Osteoarthr. Cartil. 2000, 8, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighipour, N.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Amini, S.; Amanzadeh, A.; Taghi Khorasani, M. Topological Remodeling of Cultured Endothelial Cells by Characterized Cyclic Strains. Mol. Cell Biochem. 2007, 4, 189–199. [Google Scholar]
- Henshaw, D.R.; Attia, E.; Bhargava, M.; Hannafin, J.A. Canine ACL Fibroblast Integrin Expression and Cell Alignment in Response to Cyclic Tensile Strain in Three-Dimensional Collagen Gels. J. Orthop. Res. 2006, 24, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, A.; Lim, J.; Chong, M.S.K.; Wen, F.; Liu, Y.; Pillay, Y.T.; Chan, J.K.Y.; Teoh, S.-H. In Vitro Cyclic Compressive Loads Potentiate Early Osteogenic Events in Engineered Bone Tissue: Compressive Stimulation for Bone Tissue Engineering. J. Biomed. Mater. Res. 2017, 105, 2366–2375. [Google Scholar] [CrossRef] [PubMed]
- Griensven, M.; Diederichs, S.; Roeker, S.; Boehm, S.; Peterbauer, A.; Wolbank, S.; Riechers, D.; Stahl, F.; Kasper, C. Mechanical Strain Using 2D and 3D Bioreactors Induces Osteogenesis: Implications for Bone Tissue Engineering. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Preiss-Bloom, O.; Mizrahi, J.; Elisseeff, J.; Seliktar, D. Real-Time Monitoring of Force Response Measured in Mechanically Stimulated Tissue-Engineered Cartilage. Artif. Organs 2009, 33, 318–327. [Google Scholar] [CrossRef]
- Riboh, J.; Chong, A.K.S.; Pham, H.; Longaker, M.; Jacobs, C.; Chang, J. Optimization of Flexor Tendon Tissue Engineering With a Cyclic Strain Bioreactor. J. Hand Surg. 2008, 33, 1388–1396. [Google Scholar] [CrossRef]
- Zheng, F.; Fu, F.; Cheng, Y.; Wang, C.; Zhao, Y.; Gu, Z. Organ-on-a-Chip Systems: Microengineering to Biomimic Living Systems. Small 2016, 12, 2253–2282. [Google Scholar] [CrossRef]
- Shin, S.R.; Kilic, T.; Zhang, Y.S.; Avci, H.; Hu, N.; Kim, D.; Branco, C.; Aleman, J.; Massa, S.; Silvestri, A.; et al. Label-Free and Regenerative Electrochemical Microfluidic Biosensors for Continual Monitoring of Cell Secretomes. Adv. Sci. 2017, 4, 1600522. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Elkhammas, E.; Hasan, A. Translating Advances in Organ-on-a-chip Technology for Supporting Organs. B Appl. Biomater. 2019, 107, 2006–2018. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Chen, J. The Regulation of Integrin Function by Divalent Cations. Cell Adhes. Migr. 2012, 6, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Moursi, A.; Globus, R.; Damsky, C. Interactions between Integrin Receptors and Fibronectin Are Required for Calvarial Osteoblast Differentiation in Vitro. J. Cell Sci. 1997, 110, 2187–2196. [Google Scholar] [CrossRef]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I Collagen-Induced Osteoblastic Differentiation of Bone-Marrow Cells Mediated by Collagen-α2ꞵ1 Integrin Interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 978-3-642-71033-9. [Google Scholar]
- Harburger, D.S.; Calderwood, D.A. Integrin Signalling at a Glance. J. Cell Sci. 2009, 122, 1472. [Google Scholar] [CrossRef] [Green Version]
- Geiger, B.; Yamada, K.M. Molecular Architecture and Function of Matrix Adhesions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005033. [Google Scholar] [CrossRef] [Green Version]
- Buchsbaum, R.J. Rho Activation at a Glance. J. Cell Sci. 2007, 120, 1149–1152. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell Adhesion: Integrating Cytoskeletal Dynamics and Cellular Tension. Nat. Rev. Mol. Cell Biol 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring Mechanical Tension across Vinculin Reveals Regulation of Focal Adhesion Dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix Stiffness Drives Epithelial–Mesenchymal Transition and Tumour Metastasis through a TWIST1–G3BP2 Mechanotransduction Pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-Muscle Myosin II Takes Centre Stage in Cell Adhesion and Migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a Distance: Mechanically Coupling the Extracellular Matrix with the Nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Haque, F.; Lloyd, D.J.; Smallwood, D.T.; Dent, C.L.; Shanahan, C.M.; Fry, A.M.; Trembath, R.C.; Shackleton, S. SUN1 Interacts with Nuclear Lamin A and Cytoplasmic Nesprins To Provide a Physical Connection between the Nuclear Lamina and the Cytoskeleton. Mol. Cell Biol. 2006, 26, 3738–3751. [Google Scholar] [CrossRef] [Green Version]
- Sosa, B.A.; Rothballer, A.; Kutay, U.; Schwartz, T.U. LINC Complexes Form by Binding of Three KASH Peptides to Domain Interfaces of Trimeric SUN Proteins. Cell 2012, 149, 1035–1047. [Google Scholar] [CrossRef] [Green Version]
- Dupont, S. Role of YAP/TAZ in Cell-Matrix Adhesion-Mediated Signalling and Mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ Upstream Signals and Downstream Responses. Nat. Cell Biol 2018, 20, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Jiang, J.X. Roles of Gap Junctions and Hemichannels in Bone Cell Functions and in Signal Transmission of Mechanical Stress. Front. Biosci. 2007, 12, 1450. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Liu, R.; Li, G.; Chen, M.; Shang, P.; Yang, H.; Jiang, J.X.; Xu, H. Connexin 43 Channels in Osteocytes Regulate Bone Responses to Mechanical Unloading. Front. Physiol. 2020, 11, 299. [Google Scholar] [CrossRef]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of Mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Reich, K.M.; Gay, C.V.; Frangos, J.A. Fluid Shear Stress as a Mediator of Osteoblast Cyclic Adenosine Monophosphate Production. J. Cell. Physiol. 1990, 143, 100–104. [Google Scholar] [CrossRef]
- Yue, R.; Shen, B.; Morrison, S.J. Clec11a/Osteolectin Is an Osteogenic Growth Factor That Promotes the Maintenance of the Adult Skeleton. eLife 2016, 5, e18782. [Google Scholar] [CrossRef]
- Shen, B.; Vardy, K.; Hughes, P.; Tasdogan, A.; Zhao, Z.; Yue, R.; Crane, G.M.; Morrison, S.J. Integrin Alpha11 Is an Osteolectin Receptor and Is Required for the Maintenance of Adult Skeletal Bone Mass. eLife 2019, 8, e42274. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.A.; Reina-Romo, E. Cell-Biomaterial Mechanical Interaction in the Framework of Tissue Engineering: Insights, Computational Modeling and Perspectives. IJMS 2011, 12, 8217–8244. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.; Verbruggen, S.W.; Lacroix, D. In Silico Bone Mechanobiology: Modeling a Multifaceted Biological System: In Silico Bone Mechanobiology. WIREs Syst. Biol. Med. 2016, 8, 485–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, D.R.; Wong, M. Modelling Cartilage Mechanobiology. Phil. Trans. R. Soc. Lond. B 2003, 358, 1461–1471. [Google Scholar] [CrossRef] [Green Version]
- Byrne, D.P.; Lacroix, D.; Planell, J.A.; Kelly, D.J.; Prendergast, P.J. Simulation of Tissue Differentiation in a Scaffold as a Function of Porosity, Young’s Modulus and Dissolution Rate: Application of Mechanobiological Models in Tissue Engineering. Biomaterials 2007, 28, 5544–5554. [Google Scholar] [CrossRef]
- Sanzherrera, J.; Garciaaznar, J.; Doblare, M. On Scaffold Designing for Bone Regeneration: A Computational Multiscale Approach. Acta Biomater. 2009, 5, 219–229. [Google Scholar] [CrossRef]
- Dias, M.R.; Guedes, J.M.; Flanagan, C.L.; Hollister, S.J.; Fernandes, P.R. Optimization of Scaffold Design for Bone Tissue Engineering: A Computational and Experimental Study. Med. Eng. Phys. 2014, 36, 448–457. [Google Scholar] [CrossRef]
- Halloran, J.P.; Sibole, S.; van Donkelaar, C.C.; van Turnhout, M.C.; Oomens, C.W.J.; Weiss, J.A.; Guilak, F.; Erdemir, A. Multiscale Mechanics of Articular Cartilage: Potentials and Challenges of Coupling Musculoskeletal, Joint, and Microscale Computational Models. Ann. Biomed. Eng. 2012, 40, 2456–2474. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.T. Advanced Computational Workflow for the Multi-Scale Modeling of the Bone Metabolic Processes. Med. Biol Eng Comput. 2017, 55, 923–933. [Google Scholar] [CrossRef]
- Roopavath, U.K.; Malferrari, S.; Van Haver, A.; Verstreken, F.; Rath, S.N.; Kalaskar, D.M. Optimization of Extrusion Based Ceramic 3D Printing Process for Complex Bony Designs. Mater. Des. 2019, 162, 263–270. [Google Scholar] [CrossRef]
- Conev, A.; Litsa, E.E.; Perez, M.R.; Diba, M.; Mikos, A.G.; Kavraki, L.E. Machine Learning-Guided Three-Dimensional Printing of Tissue Engineering Scaffolds. Tissue Eng. Part. A 2020, 26, 1359–1368. [Google Scholar] [CrossRef]
- Dellinger, J.G.; Wojtowicz, A.M.; Jamison, R.D. Effects of Degradation and Porosity on the Load Bearing Properties of Model Hydroxyapatite Bone Scaffolds. J. Biomed. Mater. Res. 2006, 77, 563–571. [Google Scholar] [CrossRef]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Models Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [Green Version]
- Zeiter, S.; Koschitzki, K.; Alini, M.; Jakob, F.; Rudert, M.; Herrmann, M. Evaluation of Preclinical Models for the Testing of Bone Tissue-Engineered Constructs. Tissue Eng. Part. C: Methods 2020, 26, 107–117. [Google Scholar] [CrossRef]
- Carlier, A.; Geris, L.; Lammens, J.; Van Oosterwyck, H. Bringing Computational Models of Bone Regeneration to the Clinic: Bringing Computational Models of Bone Regeneration to the Clinic. WIREs Syst. Biol. Med. 2015, 7, 183–194. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrero, S.G.; Llamas-Sillero, P.; Serrano-López, J. A Multidisciplinary Journey towards Bone Tissue Engineering. Materials 2021, 14, 4896. https://doi.org/10.3390/ma14174896
Pedrero SG, Llamas-Sillero P, Serrano-López J. A Multidisciplinary Journey towards Bone Tissue Engineering. Materials. 2021; 14(17):4896. https://doi.org/10.3390/ma14174896
Chicago/Turabian StylePedrero, Sara G., Pilar Llamas-Sillero, and Juana Serrano-López. 2021. "A Multidisciplinary Journey towards Bone Tissue Engineering" Materials 14, no. 17: 4896. https://doi.org/10.3390/ma14174896
APA StylePedrero, S. G., Llamas-Sillero, P., & Serrano-López, J. (2021). A Multidisciplinary Journey towards Bone Tissue Engineering. Materials, 14(17), 4896. https://doi.org/10.3390/ma14174896