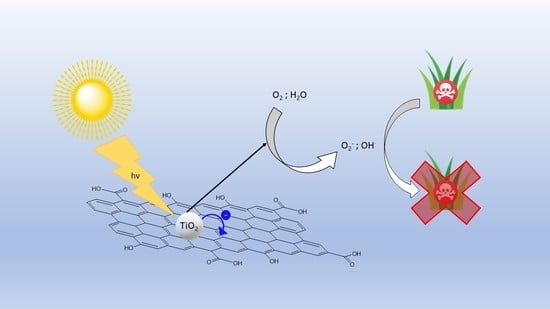
One-Pot Synthesis of TiO2-rGO Photocatalysts for the Degradation of Groundwater Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Samples Characterization
2.3. Photocatalytic Tests
2.4. Toxicity Tests
3. Results and Discussion
3.1. Materials Characterization
3.2. Photodegradation Results
3.3. Toxicity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebelo, K.; Malebo, N.; Mochane, M.J.; Masinde, M. Chemical Contamination Pathways and the Food Safety Implications along the Various Stages of Food Production: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5795. [Google Scholar] [CrossRef]
- Bunting, S.Y.; Lapworth, D.J.; Crane, E.J.; Grima-Olmedo, J.; Koroša, A.; Kuczyńska, A.; Mali, N.; Rosenqvist, L.; van Vliet, M.E.; Togola, A.; et al. Emerging organic compounds in European groundwater. Environ. Pollut. 2021, 269, 115945. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Privitera, V.; Impellizzeri, G. Selective photodegradation of 2,4-D pesticide from water by molecularly imprinted TiO2. J. Photochem. Photobiol. A Chem. 2019, 380, 111872. [Google Scholar] [CrossRef]
- Correia, F.V.; Moreira, J.C. Effects of Glyphosate and 2,4-D on Earthworms (Eisenia foetida) in Laboratory Tests. Bull. Environ. Contam. Toxicol. 2010, 85, 264–268. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Fiorenza, R.; Balsamo, S.A.; D’Urso, L.; Sciré, S.; Brundo, M.V.; Pecoraro, R.; Scalisi, E.M.; Privitera, V.; Impellizzeri, G. CeO2 for Water Remediation: Comparison of Various Advanced Oxidation Processes. Catalysts 2020, 10, 446. [Google Scholar] [CrossRef] [Green Version]
- Greco, E.; Balsamo, S.A.; Maccarrone, G.; Mello, D.; Ciliberto, E.; Shang, J.; Zhu, T. Gold-core lithium-doped titania shell nanostructures for plasmon-enhanced visible light harvesting with photocatalytic activity. J. Nanoparticle Res. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting-An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Design of graphene-based TiO2 photocatalysts-a review. Environ. Sci. Pollut. Res. 2012, 19, 3676–3687. [Google Scholar] [CrossRef]
- Ramesh, K.; Gnanavel, B.; Shkir, M. Enhanced visible light photocatalytic degradation of bisphenol A (BPA) by reduced graphene oxide (RGO)–metal oxide (TiO2, ZnO and WO3) based nanocomposites. Diam. Relat. Mater. 2021, 118, 108514. [Google Scholar] [CrossRef]
- Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. High-pressure synthesis of rGO/TiO2 and rGO/TiO2/Cu catalysts for efficient CO2 reduction under solar light. J. Supercrit. Fluids 2021, 174, 105265. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Tan, L.L.; Chai, S.P.; Mohamed, A.R. Synthesis and applications of graphene-based TiO2 photocatalysts. ChemSusChem 2012, 5, 1868–1882. [Google Scholar] [CrossRef]
- Ramesh Reddy, N.; Bhargav, U.; Mamatha Kumari, M.; Cheralathan, K.K.; Sakar, M. Review on the interface engineering in the carbonaceous titania for the improved photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 7584–7615. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. Appl. Catal. B Environ. 2015, 162, 352–363. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Wang, Y.; Tai, H.; Guo, Y. UV Illumination-enhanced molecular ammonia detection based on a ternary-reduced graphene oxide–titanium dioxide–Au composite film at room temperature. Anal. Chem. 2019, 91.5, 3311–3318. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, Y. Humidity activated ionic-conduction formaldehyde sensing of reduced graphene oxide decorated nitrogen-doped MXene/titanium dioxide composite film. Sens. Actuators B Chem. 2020, 323, 128695. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Wang, W.; Han, Q.; Zhu, Z.; Zhang, L.; Zhong, S.; Liu, B. Enhanced photocatalytic degradation performance of organic contaminants by heterojunction photocatalyst BiVO4/TiO2/RGO and its compatibility on four different tetracycline antibiotics. Adv. Powder Technol. 2019, 30, 1882–1896. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; San Martín, I.; Carrillo, P. Effect of sodium persulfate as electron acceptor on antipyrine degradation by solar TiO2 or TiO2/rGO photocatalysis. Chem. Eng. J. 2019, 364, 257–268. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.L.; Xu, D.; Huang, Y.; Wu, Z.; Wang, L.M.; Zhang, X.B. Facile, mild and fast thermal-decomposition reduction of graphene oxide in air and its application in high-performance lithium batteries. Chem. Commun. 2012, 48, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Seger, B.; Kamat, P. V UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Barakat, T.; Scirè, S.; Palmisano, L. Visible light photocatalytic activity of macro-mesoporous TiO2-CeO2 inverse opals. J. Photochem. Photobiol. A Chem. 2018, 352, 25–34. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Fiorenza, R.; Balsamo, S.A.; Condorelli, M.; D’Urso, L.; Compagnini, G.; Scirè, S. Solar photocatalytic H2 production over CeO2-based catalysts: Influence of chemical and structural modifications. Catal. Today 2021, 380, 187–198. [Google Scholar] [CrossRef]
- Sandeep, S.; Nagashree, K.; Maiyalagan, T.; Keerthiga, G. Photocatalytic degradation of 2, 4-dichlorophenoxyacetic acid—A comparative study in hydrothermal TiO2 and commercial TiO2. Appl. Surf. Sci. 2018, 449, 371–379. [Google Scholar]
- Fukshansky, L.; Remisowsky, A.M.V.; McClendon, J.; Ritterbusch, A.; Richter, T.; Mohr, H. Absorption spectra of leaves corrected for scattering and distributional error: A radiative transfer and absorption stratics treatment. Photochem. Photobiol. 1993, 57, 538–555. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Regonini, D.; Bowen, C.R.; Stevens, R. A microscopy study of the effect of heat treatment on the structure and properties of anodised TiO2 nanotubes. Appl. Surf. Sci. 2010, 256, 2672–2679. [Google Scholar] [CrossRef]
- Cheshme Khavar, A.H.; Moussavi, G.; Mahjoub, A.R. The preparation of TiO2@rGO nanocomposite efficiently activated with UVA/LED and H2O2 for high rate oxidation of acetaminophen: Catalyst characterization and acetaminophen degradation and mineralization. Appl. Surf. Sci. 2018, 440, 963–973. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Saha, A.; Moya, A.; Kahnt, A.; Iglesias, D.; Marchesan, S.; Wannemacher, R.; Prato, M.; Vilatela, J.J.; Guldi, D.M. Interfacial charge transfer in functionalized multi-walled carbon nanotube@ TiO2 nanofibres. Nanoscale 2017, 9, 7911–7921. [Google Scholar] [CrossRef] [Green Version]
- Hajialilou, E.; Asgharzadeh, H.; Khameneh Asl, S. TiO2/rGO/Cu2O ternary hybrid for high-performance photoelectrochemical applications. Appl. Surf. Sci. 2021, 544, 148832. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, Á.; Manassero, A.; Satuf, M.L.; Alfano, O.M.; Casas, J.A.; Bahamonde, A. Influence of TIO2-rGO optical properties on the photocatalytic activity and efficiency to photodegrade an emerging pollutant. Appl. Catal. B Environ. 2019, 246, 1–11. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 132–133, 452–459. [Google Scholar] [CrossRef]
- Coates, J. Encyclopedia of Analytical Chemistry -Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2004, 1–23. [Google Scholar]
- Shahriary, L.; Athawale, A.A. Graphene Oxide Synthesized by using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 02, 58–63. [Google Scholar]
- Wang, L.; Wen, M.; Wang, W.; Momuinou, N.; Wang, Z.; Li, S. Photocatalytic degradation of organic pollutants using rGO supported TiO2-CdS composite under visible light irradiation. J. Alloys Compd. 2016, 683, 318–328. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dhara, S.; Dash, S.; Tyagi, A.K. Facile gamma radiolytic methodology for TiO2-rGO synthesis: Effect on photo-catalytic H2 evolution. Int. J. Hydrogen Energy 2015, 40, 5815–5823. [Google Scholar] [CrossRef]
- Shen, T.; Wang, Q.; Guo, Z.; Kuang, J.; Cao, W. Hydrothermal synthesis of carbon quantum dots using different precursors and their combination with TiO2 for enhanced photocatalytic activity. Ceram. Int. 2018, 44, 11828–11834. [Google Scholar] [CrossRef]
- Atchudan, R.; Jebakumar Immanuel Edison, T.N.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Effective photocatalytic degradation of anthropogenic dyes using graphene oxide grafting titanium dioxide nanoparticles under UV-light irradiation. J. Photochem. Photobiol. A Chem. 2017, 333, 92–104. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Spanò, S.F.; Compagnini, G.; Sinatra, M.; D’Urso, L.; Fazio, E.; Privitera, V.; Scalese, S. Modification of graphene oxide and graphene oxide-TiO2 solutions by pulsed laser irradiation for dye removal from water. Mater. Sci. Semicond. Process. 2016, 42, 50–53. [Google Scholar] [CrossRef]
- Herring, N.P.; Almahoudi, S.H.; Olson, C.R.; El-Shall, M.S. Enhanced photocatalytic activity of ZnO-graphene nanocomposites prepared by microwave synthesis. J. Nanoparticle Res. 2012, 14. [Google Scholar] [CrossRef]
- Shi, J.; Chen, G.; Zeng, G.; Chen, A.; He, K.; Huang, Z.; Hu, L.; Zeng, J.; Wu, J.; Liu, W. Hydrothermal synthesis of graphene wrapped Fe-doped TiO2 nanospheres with high photocatalysis performance. Ceram. Int. 2018, 44, 7473–7480. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, D.; Saha, A.; Devi, P.S. Shape Transition of TiO2 Nanocube to Nanospindle Embedded on Reduced Graphene Oxide with Enhanced Photocatalytic Activity. Cryst. Growth Des. 2016, 16, 6922–6932. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Trillas, M.; Peral, J.; Domènech, X. Redox photodegradation of 2,4-dichlorophenoxyacetic acid over TiO2. Appl. Catal. B Environ. 1995, 5, 377–387. [Google Scholar] [CrossRef]
- Chenchana, A.; Nemamcha, A.; Moumeni, H.; Doña Rodríguez, J.M.; Araña, J.; Navío, J.A.; González Díaz, O.; Pulido Melián, E. Photodegradation of 2,4-dichlorophenoxyacetic acid over TiO2 (B)/anatase nanobelts and Au-TiO2 (B)/anatase nanobelts. Appl. Surf. Sci. 2019, 467–468, 1076–1087. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Maleki, A.; Rezaee, R.; Daraei, H.; Safari, M.; McKay, G.; Lee, S.M.; Jafari, A. Synthesis and Application of Fe-Doped TiO2 Nanoparticles for Photodegradation of 2,4-D from Aqueous Solution. Arab. J. Sci. Eng. 2021, 46, 6409–6422. [Google Scholar] [CrossRef]
- Lima, M.S.; Cruz-Filho, J.F.; Noleto, L.F.G.; Silva, L.J.; Costa, T.M.S.; Luz, G.E. Synthesis, characterization and catalytic activity of Fe3O4@WO3/SBA-15 on photodegradation of the acid dichlorophenoxyacetic (2,4-D) under UV irradiation. J. Environ. Chem. Eng. 2020, 8, 104145. [Google Scholar] [CrossRef]
- Pecoraro, R.; Scalisi, E.M.; Messina, G.; Fragalà, G.; Ignoto, S.; Salvaggio, A.; Zimbone, M.; Impellizzeri, G.; Brundo, M.V. Artemia salina: A microcrustacean to assess engineered nanoparticles toxicity. Microsc. Res. Tech. 2021, 84, 531–536. [Google Scholar] [CrossRef]
- Nunes, B.S.; Carvalho, F.D.; Guilhermino, L.M.; Van Stappen, G. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 2006, 144, 453–462. [Google Scholar] [CrossRef]
- Ates, M.; Daniels, J.; Arslan, Z.; Farah, I.O. Effects of aqueous suspensions of titanium dioxide nanoparticles on Artemia salina: Assessment of nanoparticle aggregation, accumulation, and toxicity. Environ. Monit. Assess. 2013, 185, 3339–3348. [Google Scholar] [CrossRef] [Green Version]
- Croghan, P.C. The Osmotic and Ionic Regulation of Artemia Salina (L.). J. Exp. Biol. 1958, 35, 219–233. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, F.; Chen, W.; Zhu, B.; Wang, G. Toxicity evaluation of graphene oxide on cysts and three larval stages of Artemia salina. Sci. Total Environ. 2017, 595, 101–109. [Google Scholar] [CrossRef]
- Cavion, F.; Fusco, L.; Sosa, S.; Manfrin, C.; Alonso, B.; Zurutuza, A.; Della Loggia, R.; Tubaro, A.; Prato, M.; Pelin, M. Ecotoxicological impact of graphene oxide: Toxic effects on the model organism Artemia franciscana. Environ. Sci. Nano 2020, 7, 3605–3615. [Google Scholar] [CrossRef]










| Photocatalyst | Isotherm | SBET1 (m2·g−1) | dP1 (nm) |
|---|---|---|---|
| TiO2 | IV; H1 | 50 | 24 |
| TiO2-GO | IV; H1 | 47 | 24 |
| TiO2-rGO thermal | IV; H3 | 44 | 18 |
| TiO2-rGO solar | IV; H2 | 53 | 6 |
| TiO2-rGO UV | IV; H2 | 55 | 5 |
| Sample | Peak D | Peak G | ID/IG ratio | FWHMD | FWHMG |
|---|---|---|---|---|---|
| GO | 1341 | 1581 | 1.05 | 143.96 ± 2.1 | 82.88 ± 1.6 |
| TiO2-GO | 1338 | 1590 | 1.05 | 191.09 ± 4.1 | 90.42 ± 2.5 |
| TiO2-rGO thermal | 1335 | 1583 | 1.13 | 145.93 ± 1.8 | 80.52 ± 1.3 |
| TiO2-rGO solar | 1340 | 1589 | 1.28 | 95.73 ± 1.3 | 67.98 ± 1.1 |
| TiO2-rGO UV | 1341 | 1592 | 1.37 | 77.2 ± 1.2 | 65.30 ± 1.3 |
| Sample | Degradation (%) | Kinetic Constant ·103 (s−1) | R2 |
|---|---|---|---|
| TiO2 | 48 | 0.06 ± 0.01 | 0.985 |
| TiO2-GO | 55 | 0.08 ± 0.03 | 0.998 |
| TiO2-rGO thermal | 63 | 0.10 ± 0.01 | 0.979 |
| TiO2-rGO solar | 97 | 0.16 ± 0.02 | 0.988 |
| TiO2-rGO UV | 97 | 0.17 ± 0.02 | 0.981 |
| pH | Kinetic Constant 103 [s−1] |
|---|---|
| 3.5 | 0.16 ± 0.02 |
| 7.2 | 0.11 ± 0.03 |
| 9.5 | 0.14 ± 0.02 |
| Sample | Experimental Setup | Degradation [%] | Main by-Products | References |
|---|---|---|---|---|
| TiO2-rGO solar 1 g/L | Artificial Solar irr., 10.7 mW/cm2, 3 h | 97 | DCP, DCR | This work |
| MI TiO2/2,4D 1 g/L | UV irr., 365 nm 12 mW/cm2, 4 h | 75 | DCP, DCR | [54] |
| TiO2 P25-Au 1 g/L | UV irr., 365 nm 9 mW/cm2, 2 h | 99 | DCP | [56] |
| Fe-doped TiO2 0.4 g/L | Artificial Solar irr., 32 mW/cm2, 2 h | 72 | - | [57] |
| Fe3O4@WO3/SBA-15 0.4 g/L | UV irr., 254 nm, 20 W, 4 h | 91 | - | [58] |
| Sample | CTRL | 10−1 | 10−2 | 10−3 | 10−4 |
|---|---|---|---|---|---|
| TiO2-1%rGO 24 h | 0% | 1.56 % | 4.16% | 5.73% | 6.77% |
| TiO2-1%rGO 48 h | 0.52% | 2.60% | 5.21% | 7.81% | 8.85% |
| TiO2-2%rGO 24 h | 0% | 2.08% | 2.08% | 4.69% | 2.08% |
| TiO2-2%rGO 48 h | 0.52% | 5.73% | 5.73% | 6.77% | 5.21% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balsamo, S.A.; Fiorenza, R.; Condorelli, M.; Pecoraro, R.; Brundo, M.V.; Lo Presti, F.; Sciré, S. One-Pot Synthesis of TiO2-rGO Photocatalysts for the Degradation of Groundwater Pollutants. Materials 2021, 14, 5938. https://doi.org/10.3390/ma14205938
Balsamo SA, Fiorenza R, Condorelli M, Pecoraro R, Brundo MV, Lo Presti F, Sciré S. One-Pot Synthesis of TiO2-rGO Photocatalysts for the Degradation of Groundwater Pollutants. Materials. 2021; 14(20):5938. https://doi.org/10.3390/ma14205938
Chicago/Turabian StyleBalsamo, Stefano Andrea, Roberto Fiorenza, Marcello Condorelli, Roberta Pecoraro, Maria Violetta Brundo, Francesca Lo Presti, and Salvatore Sciré. 2021. "One-Pot Synthesis of TiO2-rGO Photocatalysts for the Degradation of Groundwater Pollutants" Materials 14, no. 20: 5938. https://doi.org/10.3390/ma14205938
APA StyleBalsamo, S. A., Fiorenza, R., Condorelli, M., Pecoraro, R., Brundo, M. V., Lo Presti, F., & Sciré, S. (2021). One-Pot Synthesis of TiO2-rGO Photocatalysts for the Degradation of Groundwater Pollutants. Materials, 14(20), 5938. https://doi.org/10.3390/ma14205938