Clinical Evaluation of Dental Implants with a Double Acid-Etched Surface Treatment: A Cohort Observational Study with Up to 10-Year Follow-Up
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Parameters
- Probing Depth (PD): a periodontal probe cp-15 (Hu-Friedy®, Chicago, IL, USA), calibrated at intervals of 1 mm, was used to evaluate peri-implant mucosa behavior (Figure 3). PDs of 0–6 mm were considered physiological, and, when >6 mm, as pathological. Three points on vestibular and lingual/palatal aspects were evaluated for each implant;
- 2.
- To assess implant stability, resonance frequency analysis (RFA) produced an ISQ value for each implant. Evaluations were performed at different times after loading, depending on group. RFA was measured with the Ostell® device (Malmö, Sweden), obtaining ISQ values on a scale of 1 to 100 (kHz). To obtain this, each patient’s implant-supported prosthesis was removed (prosthesis screwed to the implant in all cases) and implant stability was measured. The crown was then screwed back into the dental implant, applying a torque of 30 Ncm;
- 3.
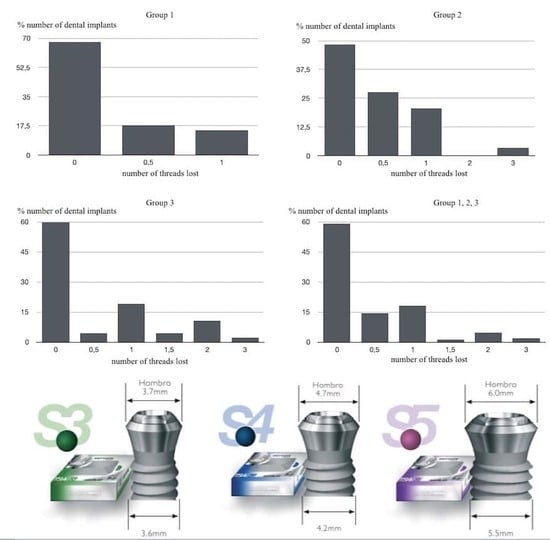
- Marginal Bone Loss (MBL): periapical intraoral radiographs were used to evaluate bone loss around the implants (Sirona Heliodent with 1 mm aluminum filter). A Rinn Endoray® plate holder (Markham, ON, Canada) was used to maintain the same object focus distance for all patients, and to avoid focus distance variations that could lead to inaccurate results. The distance between the bone crest and the implant shoulder on both mesial and distal aspects was measured from periapical radiographs by the same operator in all cases. These measurements were expressed by the number of implant threads lost (Figure 4).
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BIC | Bone Implant Contact |
| MBL | Marginal Bone Loss |
| RFA | Resonance frequency analysis |
| ISQ | Implant stability quotient |
| PD | Probing Depth |
References
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Edelmayer, M.; Woletz, K.; Ulm, C.; Zechner, W.; Tepper, G. Patient information on treatment alternatives for missing single teeth—Systematic review. Eur. J. Oral Implant. 2016, 9, S45–S57. [Google Scholar] [CrossRef] [Green Version]
- Del Fabbro, M.; Testori, T.; Kekovic, V.; Goker, F.; Tumedei, M.; Wang, H.L. A Systematic Review of Survival Rates of Osseointegrated Implants in Fully and Partially Edentulous Patients Following Immediate Loading. J. Clin. Med. 2019, 8, 2142. [Google Scholar] [CrossRef] [Green Version]
- Mertens, C.; Steveling, H.G.; Stucke, K.; Pretzl, B.; Meyer-Bäumer, A. Fixed Implant-Retained Rehabilitation of the Edentulous Maxilla: 11-Year Results of a Prospective Study. Clin. Implant. Dent. Relat. Res. 2012, 14, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Bonino, L.; Dalmasso, P.; Aglietta, M. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin. Oral Implant. Res. 2013, 25, 1105–1112. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef]
- Blatt, S.; Pabst, A.M.; Schiegnitz, E.; Hosang, M.; Ziebart, T.; Walter, C.; Al-Nawas, B.; Klein, M.O. Early cell response of osteogenic cells on differently modified implant surfaces: Sequences of cell proliferation, adherence and differentiation. J. Cranio-Maxillofac. Surg. 2017, 46, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; De Lima, J.H.C.; Rodriguez, F.; Calvo-Guirado, J.L.; Júnior, J.A.; Pérez-Díaz, L.; Mazón, P.; Aragoneses, J.M.; De Aza, P.N. Microgrooves and Microrugosities in Titanium Implant Surfaces: An In Vitro and In Vivo Evaluation. Materials 2019, 12, 1287. [Google Scholar] [CrossRef] [Green Version]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the Titanium Implant Surface Treatment on the Surface Roughness and Chemical Composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef] [Green Version]
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-term implant survival and success: A 10–16-year follow-up of non-submerged dental implants. Clin. Oral Implant. Res. 2010, 21, 772–777. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Jimenez-Guerra, A.; Monsalve-Guil, L.; Ortiz-Garcia, I.; Nicolas-Silvente, A.I.; Segura-Egea, J.J.; Lopez-Lopez, J. Long-Term Clinical Outcomes of Treatment with Dental Implants with Acid Etched Surface. Materials 2020, 13, 1553. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Crocetta, E.; Quaranta, A.; Lorusso, F. Influence of the Thermal Treatment to Address a Better Osseointegration of Ti6Al4V Dental Implants: Histological and Histomorphometrical Study in a Rabbit Model. BioMed Res. Int. 2018, 2018, 2349698. [Google Scholar] [PubMed] [Green Version]
- Peñarrocha, M.; Carrillo, C.; Boronat, A.; Martí, E. Early Loading of 642 Defcon Implants: 1-Year Follow-Up. J. Oral Maxillofac. Surg. 2007, 65, 2317–2320. [Google Scholar] [CrossRef] [PubMed]
- Barona-Dorado, C.; Martínez-Rodríguez, N.; Torres-Lear, F.; Martínez-González, J.-M. Observational study of 67 wide platform implants treated with avantblast surface. Results at three year. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, E183–E187. [Google Scholar]
- Gursoytrak, B.; Ataoglu, H. Use of resonance frequency analysis to evaluate the e_ects of surface propertieson the stability of di_erent implants. Clin. Oral Implant. Res. 2019, 31, 239–245. [Google Scholar] [CrossRef]
- Malchiodi, L.; Ghensi, P.; Cucchi, A.; Trisi, P.; Szmukler-Moncler, S.; Corrocher, G.; Gerosa, R. Early bone formation around immediately loaded FBR-coated implants after 8, 10 and 12 weeks: A human histologic evaluation of three retrieved implants. Minerva Stomatol. 2011, 60, 205–216. [Google Scholar] [PubMed]
- Mistry, S.; Kundu, D.; Datta, S.; Basu, D. Comparison of bioactive glass coated and hydroxyapatite coated titanium dental implants in the human jaw bone. Aust. Dent. J. 2011, 56, 68–75. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; De Sousa, B.M.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef] [PubMed]
- Göthberg, C.; Gröndahl, K.; Omar, O.; Thomsen, P.; Slotte, C. Bone and soft tissue outcomes, risk factors, and complications of implant-supported prostheses: 5-Years RCT with different abutment types and loading protocols. Clin. Implant. Dent. Relat. Res. 2018, 20, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Garcia, A.; Castellanos-Cosano, L.; Corcuera-Flores, J.R.; Rodriguez-Perez, A.; Torres-Lagares, D.; Machuca-Portillo, G. Influence of marginal bone loss on peri-implantitis: Systematic review of literature. J. Clin. Exp. Dent. 2019, 11, e1045–e1071. [Google Scholar] [CrossRef]
- Cochran, D.L.; Jackson, J.M.; Bernard, J.-P.; Bruggenkate, C.M.T.; Buser, D.; Taylor, T.D.; Weingart, D.; Schoolfield, J.D.; Jones, A.A.; Oates, T.W. A 5-year prospective multicenter study of early loaded titanium implants with a sandblasted and acid-etched surface. Int. J. Oral Maxillofac. Implant. 2011, 26, 1324–1332. [Google Scholar]
- Jung, R.E.; Zembic, A.; Pjetursson BEZwahlen, M.; Thomas, D.S. Systematic review of the survival rate and the incidence of biological technical and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implant. Res. 2012, 23, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Linkevicius, R.; Alkimavicius, J.; Linkeviciene, L.; Andrijauskas, P.; Puisys, A. Influence of titanium base, lithium disilicate restoration and vertical soft tissue thickness on bone stability around triangular-shaped implants: A prospective clinical trial. Clin. Oral Implant. Res. 2018, 29, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Puisys, A.; Linkevicius, R.; Dds, J.A.; Gineviciute, E.; Dds, L.L. The influence of submerged healing abutment or subcrestal implant placement on soft tissue thickness and crestal bone stability. A 2-year randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2020, 22, 497–506. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; López-López, P.J.; Martínez, C.P.-A.; Javed, F.; Granero-Marin, J.M.; De Val, J.E.M.S.; Fernández, M.P.R. Peri-implant bone loss clinical and radiographic evaluation around rough neck and microthread implants: A 5-year study. Clin. Oral Implant. Res. 2016, 29, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Kesar, N.; Begić, A.; Von Krockow, N.; Nentwig, G.; Weigl, P. Incidence and prevalence of peri-implantitis and peri-implant mucositis 17 to 23 (18.9) years postimplant placement. Clin. Implant. Dent. Relat. Res. 2019, 21, 1116–1123. [Google Scholar] [CrossRef]
- Farina, R.; Filippi, M.; Brazzioli, J.; Tomasi, C.; Trombelli, L. Bleeding on probing around dental implants: A retrospective study of associated factors. J. Clin. Periodontol. 2016, 44, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Munoz, V.; Duque, A.; Giraldo, A.; Manrique, R. Prevalence of Peri-implant Disease According to Periodontal Probing Depth and Bleeding on Probing: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, e89–e105. [Google Scholar]
- Longoni, S.; Tinto, M.; Pacifico, C.; Sartori, M.; Andreano, A. Effect of Peri-implant Keratinized Tissue Width on Tissue Health and Stability: Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2019, 34, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-Y.; Ni, J.; Zhuang, L.-F.; Zhang, X.-M.; Fan, L.-F.; Lai, H.-C. Peri-implant conditions and marginal bone loss around cemented and screw-retained single implant crowns in posterior regions: A retrospective cohort study with up to 4 years follow-up. PLoS ONE 2018, 13, e0191717. [Google Scholar] [CrossRef]
- Froum, S.J.; Khouly, I. Survival Rates and Bone and Soft Tissue Level Changes Around One-Piece Dental Implants Placed with a Flapless or Flap Protocol: 8.5-Year Results. Int. J. Periodontics Restor. Dent. 2017, 37, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Rammelsberg, P.; Kappel, S.; Lorenzo-Bermejo, J. Effect of prosthetic restoration on implant survival and success. Clin. Oral Implant. Res. 2016, 28, 1296–1302. [Google Scholar] [CrossRef]
- Msc, F.S.L.; Douglas-De-Oliveira, D.W.; Costa, F. Relationship between implant stability measurements obtained by insertion torque and resonance frequency analysis: A systematic review. Clin. Implant. Dent. Relat. Res. 2017, 20, 26–33. [Google Scholar] [CrossRef]
- Tanaka, K.; Sailer, I.; Iwama, R.; Yamauchi, K.; Nogami, S.; Yoda, N.; Takahashi, T. Relationship between cortical bone thickness and implant stability at the time of surgery and secondary stability after osseointegration measured using resonance frequency analysis. J. Periodontal Implant. Sci. 2018, 48, 360–372. [Google Scholar] [CrossRef]
- Buser, D.; Weber, H.P.; Bragger, U.; Balsiger, C. Tissue integration of one-stage ITI implants: 3-year results of a longitudinal study with Hollow-Cylinder and Hollow-Screw implants. Int. J. Oral Maxillofac. Implant. 1991, 6, 405–412. [Google Scholar] [CrossRef]
- Fischer, K.; Bäckström, M.; Sennerby, L. Immediate and Early Loading of Oxidized Tapered Implants in the Partially Edentulous Maxilla: A 1-Year Prospective Clinical, Radiographic, and Resonance Frequency Analysis Study. Clin. Implant. Dent. Relat. Res. 2009, 11, 69–80. [Google Scholar] [CrossRef]
- Chen, M.H.-M.; Lyons, K.; Tawse-Smith, A.; Ma, S. Resonance Frequency Analysis in Assessing Implant Stability: A Retrospective Analysis. Int. J. Prosthodont. 2019, 32, 317–326. [Google Scholar] [CrossRef]
- Rodrigo, D.; Sanz-Sánchez, I.; Figuero, E.; Llodrá, J.C.; Bravo, M.; Caffesse, R.G.; Vallcorba, N.; Guerrero, A.; Herrera, D. Prevalence and risk indicators of peri-implant diseases in Spain. J. Clin. Periodontol. 2018, 45, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Pjetursson, B.E.Z.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar] [CrossRef]
- López-Valverde, N.; Pardal-Peláez, B.; López-Valverde, A.; Flores-Fraile, J.; Herrero-Hernández, S.; Macedo-De-Sousa, B.; Herrero-Payo, J.; Ramírez, J. Effectiveness of Propolis in the Treatment of Periodontal Disease: Updated Systematic Review with Meta-Analysis. Antioxidants 2021, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Comino-Garayoa, R.; Brinkmann, J.C.-B.; Peláez, J.; López-Suárez, C.; Martínez-González, J.M.; Suárez, M.J. Allergies to Titanium Dental Implants: What Do We Really Know about Them? A Scoping Review. Biology 2020, 9, 404. [Google Scholar] [CrossRef]
- Mangano, C.; Mangano, F.G.; Shibli, J.A.; Roth, L.A.; Addazio, G.D.; Piattelli, A.; Iezzi, G. Immunohistochemical Evaluation of Peri-Implant Soft Tissues around Machined and Direct Metal Laser Sintered (DMLS) Healing Abutments in Humans. Int. J. Environ. Res. Public Heal. 2018, 15, 1611. [Google Scholar] [CrossRef] [Green Version]
- Dellavia, C.; Canullo, L.; Allievi, C.; Lang, N.P.; Pellegrini, G. Soft tissue surrounding switched platform implants: An immunohistochemical evaluation. Clin. Oral Implant. Res. 2011, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2016, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Lew, W.-Z.; Lu, E.; Loretz, T.; Lu, L.; Lin, C.-T.; Feng, S.-W. An evaluation of the biocompatibility and osseointegration of novel glass fiber reinforced composite implants: In vitro and in vivo studies. Dent. Mater. 2018, 34, 470–485. [Google Scholar] [CrossRef]







| Implant Characteristics | n = 110 | |||
|---|---|---|---|---|
| Diameter | S3 (3.6 mm) nº implants: 30 (27%) | S4 (4.2 mm) nº implants: 50 (46%) | S5 (5.5 mm) nº implants: 30 (27%) | |
| Length | 10 mm nº implants: 28 (25.5%) | 11.5 mm nº implants: 34 (30.9%) | 13 mm nº implants: 36 (32.7%) | 14.5 mm nº implants: 12 (10.9%) |
| anterior sector (incisor-canine) | premolar sector (premolars) | posterior sector (molars) | ||
| Location | nº maxilla: 23 (20.9%) nºmandible: 1 (0.9%) | nº maxilla: 39 (35.5%) nº mandible: 13 (11.8%) | nº maxilla: 6 (5.5%) nº mandible: 28 (25.5%) | |
| Time of loading | from 1 to 3 years nº implants: 28 (25.2%) | from 3 to 5 years nº implants: 70 (63.96%) | more than 5 years nº implants: 12 (10.81%) |
| Authors | Year | Study Type | Nº Patients | Age (Years) | Nº Implants | Implant System | MBL (mm) |
|---|---|---|---|---|---|---|---|
| Cochran et al. [23] | 2011 | Prospective multicenter | 135 | 55 | n = 439 | St SLA | NR |
| Rammelsberg et al. [35] | 2017 | Prospective | 630 | 59.56 | n = 1569 | St TL, St BL, Nb Replace | NR |
| Froum et al. [34] | 2017 | Cohorts | 28 | 56.8y | n = 28 | Nb | 0.848 |
| Shi et al. [33] | 2018 | Retrospective cohort | 176 | 49.6 SG; 46.8 CG | n = 176 | St TL | 3.5 |
| Calvo-Guirado et al. [27] | 2018 | Prospective | 53 | 37.85 | n = 71 | MIS | 0.90 |
| Chen et al. [40] | 2019 | Retrospective analysis | 173 | 21–85 | n = 383 | NR | 0.03 ± 0.091 |
| Our study | 2021 | Observational | 110 | 50.85 | n = 111 | Phibo® TSA | 0.38 |
| Authors | RFA (mean ISQ) | Probing depth (mm) | Survival rate (%) | Success rate (%) | Restoration | follow-up (years) | |
| Cochran et al. [23] | NR | 2.7 | 99.1 | 98.8 | NR | 5 | |
| Rammelsberg et al. [35] | NR | NR | NR | 97 (single crowns) | NR | 5.0 | |
| Froum et al. [34] | NR | 2.089 | 100 | 96.4 | NR | 8.5 | |
| Shi et al. [33] | NR | 3.78(SG); 3.43(CG) | 100 SG, 98.8 CG | NR | CG, SG | 2.5 | |
| Calvo-Guirado et al. [27] | NR | NR | 100 % | NR | CG, SG | 5 | |
| Chen et al. [40] | 65.5 ± 6.9 | NR | 95 | NR | 10 | ||
| Our study | 65.54 | 3.4 mm | 99.1 | 96.37 | SG | From 1 to 10 years | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos Marino, J.; Cortés-Bretón Brinkmann, J.; García-Gil, I.; Martínez-Rodríguez, N.; Fraile, J.F.; Barona Dorado, C.; Martínez-González, J.M. Clinical Evaluation of Dental Implants with a Double Acid-Etched Surface Treatment: A Cohort Observational Study with Up to 10-Year Follow-Up. Materials 2021, 14, 6483. https://doi.org/10.3390/ma14216483
Santos Marino J, Cortés-Bretón Brinkmann J, García-Gil I, Martínez-Rodríguez N, Fraile JF, Barona Dorado C, Martínez-González JM. Clinical Evaluation of Dental Implants with a Double Acid-Etched Surface Treatment: A Cohort Observational Study with Up to 10-Year Follow-Up. Materials. 2021; 14(21):6483. https://doi.org/10.3390/ma14216483
Chicago/Turabian StyleSantos Marino, Juan, Jorge Cortés-Bretón Brinkmann, Ignacio García-Gil, Natalia Martínez-Rodríguez, Javier Flores Fraile, Cristina Barona Dorado, and José María Martínez-González. 2021. "Clinical Evaluation of Dental Implants with a Double Acid-Etched Surface Treatment: A Cohort Observational Study with Up to 10-Year Follow-Up" Materials 14, no. 21: 6483. https://doi.org/10.3390/ma14216483
APA StyleSantos Marino, J., Cortés-Bretón Brinkmann, J., García-Gil, I., Martínez-Rodríguez, N., Fraile, J. F., Barona Dorado, C., & Martínez-González, J. M. (2021). Clinical Evaluation of Dental Implants with a Double Acid-Etched Surface Treatment: A Cohort Observational Study with Up to 10-Year Follow-Up. Materials, 14(21), 6483. https://doi.org/10.3390/ma14216483