Evaluation of Injectable Hyaluronic Acid-Based Hydrogels for Endodontic Tissue Regeneration
Abstract
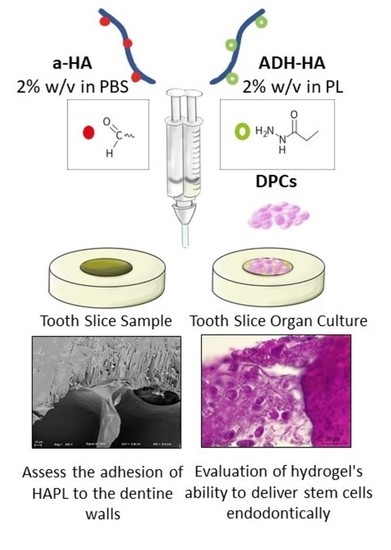
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrogels Precursors
2.1.1. Hydrazide-Modified Hyaluronic Acid (ADH-HA)
2.1.2. Aldehyde-Modified HA (a-HA)
2.1.3. Platelet Lysate (PL)
2.1.4. Preparation of PL-Laden HA Hydrogels (HAPL)
2.2. Characterization of Hydrogels
Working Time and Setting Time
2.3. Preparation of Tooth Slice Samples
2.4. Assess the Adhesion of HAPL to the Dentine Walls
2.4.1. Scanning Electron Microscopy (SEM) Analysis
2.4.2. Tensile Test
2.4.3. Indentation Test
2.5. Evaluation of Hydrogel’s Ability to Deliver Stem Cells Endodontically
2.5.1. Expansion of Human Dental Tissues Derived Cells
2.5.2. Encapsulation of DPCs
2.5.3. Tooth Slice Organ Cultures
2.5.4. Fluorescence Microscopy
2.5.5. Histological Processing
2.6. Statistical Analysis
3. Results
3.1. Working Time (wT) and Setting Time (sT)
3.2. Dentin Pre-Conditioning and Hydrogel Microstructure
3.3. Ability of the Hydrogels to Adhere to the Dentine Walls
3.4. Hydrogel’s Ability to Deliver Dental Derived Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boykin, M.J.; Gilbert, G.H.; Tilashalski, K.R.; Shelton, B.J. Incidence of Endodontic Treatment: A 48-Month Prospective Study. J. Endod. 2003, 29, 806–809. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Schmalz, G.; Smith, A.J. Pulp Development, Repair, and Regeneration: Challenges of the Transition from Traditional Dentistry to Biologically Based Therapies. J. Endod. 2014, 40, S2–S5. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Berman, L.H. Cohen’s Pathways of the Pulp, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Fransson, H.; Dawson, V.S.; Frisk, F.; Bjørndal, L.; Kvist, T.; Jonasson, P.; Markvart, M.; Petersson, K.; Pigg, M.; Reit, C.; et al. Survival of Root-filled Teeth in the Swedish Adult Population. J. Endod. 2016, 42, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Borén, D.L.; Jonasson, P.; Kvist, T. Long-term Survival of Endodontically Treated Teeth at a Public Dental Specialist Clinic. J. Endod. 2015, 41, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Clinical Perspectives of Pulp Regeneration. J. Endod. 2020, 46, S161–S174. [Google Scholar] [CrossRef]
- Shrestha, S.; Diogenes, A.; Kishen, A. Temporal-controlled Release of Bovine Serum Albumin from Chitosan Nanoparticles: Effect on the Regulation of Alkaline Phosphatase Activity in Stem Cells from Apical Papilla. J. Endod. 2014, 40, 1349–1354. [Google Scholar] [CrossRef]
- Marí-Beffa, M.; Segura-Egea, J.J.; Díaz-Cuenca, A. Regenerative Endodontic Procedures: A Perspective from Stem Cell Niche Biology. J. Endod. 2017, 43, 52–62. [Google Scholar] [CrossRef]
- Lin, C.; Ekblad-Nordberg, Å.; Michaëlsson, J.; Götherström, C.; Hsu, C.-C.; Ye, H.; Johansson, J.; Rising, A.; Sundström, E.; Åkesson, E. In Vitro Study of Human Immune Responses to Hyaluronic Acid Hydrogels, Recombinant Spidroins and Human Neural Progenitor Cells of Relevance to Spinal Cord Injury Repair. Cells 2021, 10, 1713. [Google Scholar] [CrossRef]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Park, J.Y.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. An injectable click-crosslinked hyaluronic acid hydrogel modified with a BMP-2 mimetic peptide as a bone tissue engineering scaffold. Acta Biomater. 2020, 117, 108–120. [Google Scholar] [CrossRef]
- Banerjee, A.; Koul, V.; Bhattacharyya, J. Fabrication of In Situ Layered Hydrogel Scaffold for the Co-delivery of PGDF-BB/Chlorhexidine to Regulate Proinflammatory Cytokines, Growth Factors, and MMP-9 in a Diabetic Skin Defect Albino Rat Model. Biomacromolecules 2021, 22, 1885–1900. [Google Scholar] [CrossRef]
- Berman, L.H.; Hargreaves, K.M. Cohen’s Pathway of the Pulp; Elsevier: St. Louis, MO, USA, 2020. [Google Scholar]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Babo, P.; Santo, V.E.; Duarte, A.R.C.; Correia, C.; Costa, M.H.G.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Platelet lysate membranes as new autologous templates for tissue engineering applications. Inflamm. Regen. 2014, 34, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.D.F.; Babo, P.S.; Silva, C.R.; Rodrigues, M.T.; Hebling, J.; Reis, R.L.; Gomes, M.E. Hyaluronic acid hydrogels incorporating platelet lysate enhance human pulp cell proliferation and differentiation. J. Mater. Sci. Mater. Med. 2018, 29, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Xia, Y.; Qiu, Y.; Chen, X.; Shi, S. Preparation and property of starch nanoparticles reinforced aldehyde-hydrazide covalently crosslinked PNIPAM hydrogels. J. Appl. Polym. Sci. 2017, 135, 45761. [Google Scholar] [CrossRef]
- Mendes, B.; Gómez-Florit, M.; Araújo, A.C.; Prada, J.; Babo, P.S.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Intrinsically Bioactive Cryogels Based on Platelet Lysate Nanocomposites for Hemostasis Applications. Biomacromolecules 2020, 21, 3678–3692. [Google Scholar] [CrossRef]
- Sloan, A.; Shelton, R.; Hann, A.; Moxham, B.; Smith, A. An in vitro approach for the study of dentinogenesis by organ culture of the dentine–pulp complex from rat incisor teeth. Arch. Oral Biol. 1998, 43, 421–430. [Google Scholar] [CrossRef]
- Saw, T.Y.; Cao, T.; Yap, A.U.J.; Ng, M.M.L. Tooth slice organ culture and established cell line culture models for cytotoxicity assessment of dental materials. Toxicol. Vitr. 2005, 19, 145–154. [Google Scholar] [CrossRef]
- Barros, V.; Gomes, M.E.; Dias, I.R. Contribution to the Characterization of the Glucocorticoid Treated Ovariectomized Os-teoporotic Sheep Model for Pre-Clinical and Translational Studies in Orthopaedic Research- Bone Mineral Density and His-tological and Histomorphometric Analysis. Ph.D. Thesis, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal, 2018. [Google Scholar]
- Xu, J.; Liu, Y.; Hsu, S.-H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical Properties of 5 Root Canal Sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef]
- Kulchat, S.; Chaur, M.N.; Lehn, J. Kinetic Selectivity and Thermodynamic Features of Competitive Imine Formation in Dynamic Covalent Chemistry. Chem.-A Eur. J. 2017, 23, 11108–11118. [Google Scholar] [CrossRef]
- Trope, M.; Bunes, A.; Debelian, G. Root filling materials and techniques: Bioceramics a new hope? Endod. Top. 2015, 32, 86–96. [Google Scholar] [CrossRef]
- Goldberg, F.; Gurfinkel, J. Analysis of the use of Dycal with gutta-percha points as an endodontic filling technique. Oral Surg. Oral Med. Oral Pathol. 1979, 47, 78–82. [Google Scholar] [CrossRef]
- Stanley, H.R.; Pameijer, C.H. Pulp capping with a new visible-light-curing calcium hydroxide composition (Prisma VLC Dycal). Oper. Dent. 1985, 10, 156–163. [Google Scholar] [PubMed]
- Louf, J.-F.; Lu, N.B.; O’Connell, M.G.; Cho, H.J.; Datta, S.S. Under pressure: Hydrogel swelling in a granular medium. Sci. Adv. 2021, 7, eabd2711. [Google Scholar] [CrossRef]
- Wiesse, P.E.B.; Silva-Sousa, Y.T.; Pereira, R.D.; Estrela, C.; Domingues, L.M.; Pécora, J.D.; Sousa-Neto, M.D. Effect of ultrasonic and sonic activation of root canal sealers on the push-out bond strength and interfacial adaptation to root canal dentine. Int. Endod. J. 2018, 51, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meza, G.; Urrejola, D.; Jean, N.S.; Inostroza, C.; López, V.; Khoury, M.; Brizuela, C. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J. Endod. 2019, 45, 144–149. [Google Scholar] [CrossRef]
- Hanson, A.J.; Quinn, M.T. Effect of fibrin sealant composition on human neutrophil chemotaxis. J. Biomed. Mater. Res. 2002, 61, 474–481. [Google Scholar] [CrossRef]
- Wang, F.-M.; Qiu, K.; Hu, T.; Wan, C.-X.; Zhou, X.-D.; Gutmann, J.L. Biodegradable porous calcium polyphosphate scaffolds for the three-dimensional culture of dental pulp cells. Int. Endod. J. 2006, 39, 477–483. [Google Scholar] [CrossRef]
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion–not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Babo, P.S.; Pires, R.L.; Santos, L.; Franco, A.; Rodrigues, F.; Leonor, I.; Reis, R.L.; Gomes, M.E. Platelet Lysate-Loaded Photocrosslinkable Hyaluronic Acid Hydrogels for Periodontal Endogenous Regenerative Technology. ACS Biomater. Sci. Eng. 2017, 3, 1359–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Xu, G.; Gao, Z.; Liu, Z.; Xu, J.; Wang, J.; Zhang, C.; Wang, S. Demineralized Dentin Matrix Induces Odontoblastic Differentiation of Dental Pulp Stem Cells. Cells Tissues Organs 2016, 201, 65–76. [Google Scholar] [CrossRef]
- Salehi, S.; Cooper, P.; Smith, A.; Ferracane, J. Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dent. Mater. 2016, 32, 334–342. [Google Scholar] [CrossRef]
- Huang, G.T.-J.; Shagramanova, K.; Chan, S.W. Formation of Odontoblast-Like Cells from Cultured Human Dental Pulp Cells on Dentin In Vitro. J. Endod. 2006, 32, 1066–1073. [Google Scholar] [CrossRef]
- Lobat, T. Applications of Biomedical Engineering in Dentistry; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Domingues, R.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.; Mano, J.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjug. Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef]
- Patil, R.; Kansara, V.; Ray, D.; Aswal, V.K.; Jha, P.K.; Bahadur, P.; Tiwari, S. Slow degrading hyaluronic acid hydrogel reinforced with cationized graphene nanosheets. Int. J. Biol. Macromol. 2019, 141, 232–239. [Google Scholar] [CrossRef]
- Nair, P.N.R. Critical Reviews in Oral Biology & Medicine and the causes of endodontic failures. Int. Am. Assoc. Dent. Res. 2004, 15, 348–381. [Google Scholar]
- Koppang, H.S.; Koppang, R.; Solheim, T.; Aarnes, H.; Stølen, S. Ørbeck Cellulose fibers from endodontic paper points as an etiological factor in postendodontic periapical granulomas and cysts. J. Endod. 1989, 15, 369–372. [Google Scholar] [CrossRef]





| Material | Test | N | Mean (s) | SD (s) | Min (s) | Max (s) |
|---|---|---|---|---|---|---|
| Control | wT | 5 | 10.0 | 1.4 | 8 | 12 |
| HAPL | wT | 5 | 138.8 | 29.6 | 105 | 178 |
| Control | sT | 5 | 20.8 | 1.6 | 19 | 23 |
| HAPL | sT | 5 | 373.4 | 89.1 | 302 | 516 |
| Test | Material | Measure | 1 | 2 | 3 | 4 | 5 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|
| Tensile | Control | Displacement (mm) | 1.1412 | 0.8674 | 0.8643 | 0.9301 | 0.7943 | 0.9194 | 0.1329 |
| Tensile stress (MPa) | 0.0061 | 0.0061 | 0.0043 | 0.0043 | 0.0082 | 0.0058 | 0.0016 | ||
| HAPL | Displacement (mm) | 5.7341 | 2.5421 | 4.0172 | 6.6927 | 2.5673 | 4.3106 | 1.8677 | |
| Tensile stress (MPa) | 0.0004 | 0.0009 | 0.0009 | 0.0005 | 0.0009 | 0.0007 | 0.0002 | ||
| Indentation | Control | Displacement (mm) | 0.2333 | 0.2815 | 0.2266 | 0.2906 | 0.3142 | 0.2692 | 0.0379 |
| Compressive stress (kPa) | 0.0040 | 0.0054 | 0.0031 | 0.0055 | 0.0038 | 0.0043 | 0.0010 | ||
| HAPL | Displacement (mm) | 1.0541 | 1.6003 | 1.0902 | 1.6001 | 1.6834 | 1.4056 | 0.3065 | |
| Compressive stress (kPa) | 3.9074 | 4.6874 | 5.2862 | 6.5021 | 7.4143 | 5.5594 | 1.4047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astudillo-Ortiz, E.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Evaluation of Injectable Hyaluronic Acid-Based Hydrogels for Endodontic Tissue Regeneration. Materials 2021, 14, 7325. https://doi.org/10.3390/ma14237325
Astudillo-Ortiz E, Babo PS, Reis RL, Gomes ME. Evaluation of Injectable Hyaluronic Acid-Based Hydrogels for Endodontic Tissue Regeneration. Materials. 2021; 14(23):7325. https://doi.org/10.3390/ma14237325
Chicago/Turabian StyleAstudillo-Ortiz, Esteban, Pedro S. Babo, Rui L. Reis, and Manuela E. Gomes. 2021. "Evaluation of Injectable Hyaluronic Acid-Based Hydrogels for Endodontic Tissue Regeneration" Materials 14, no. 23: 7325. https://doi.org/10.3390/ma14237325
APA StyleAstudillo-Ortiz, E., Babo, P. S., Reis, R. L., & Gomes, M. E. (2021). Evaluation of Injectable Hyaluronic Acid-Based Hydrogels for Endodontic Tissue Regeneration. Materials, 14(23), 7325. https://doi.org/10.3390/ma14237325