Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
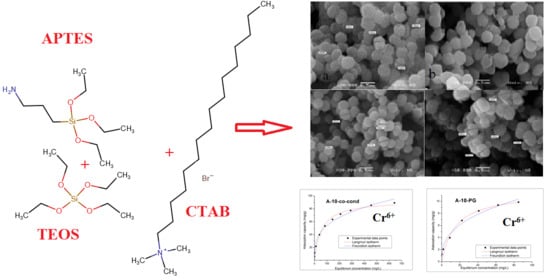
2.1.1. Synthesis of Functionalized Mesoporous Materials via Co-Condensation Method
2.1.2. Synthesis of Functionalized Mesoporous Materials via Post-Grafting
2.2. Characterization Methods
3. Results and Discussions
3.1. FT-IR
3.2. Nitrogen Adsorption Measurements
3.3. Small angle X-ray Scattering (SAXS) and Small Angle Neutron Scattering (SANS) Analysis of the Particle Morphology and Pore Structure
3.4. SEM Analysis
3.5. Sorption Studies
3.5.1. Zeta Potential Measurements
3.5.2. Point of Zero Charge (pZc) Determined by Using the Method of Bringing to Equilibrium
3.5.3. Batch Adsorption Studies
Effect of pH
Contact Time and Temperature Effect. Kinetic and Thermodynamic Studies
Adsorption Isotherms
3.6. Comparison with Other Adsorbents for Cr(VI) Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Miao, J.; Shuai, Q. Carboxyl-functionalized magnetic porous organic polymers as efficient adsorbent for wastewater remediation. J. Taiwan Inst. Chem. Eng. 2020, 109, 97–102. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.; Wang, X.; Yin, X. Efficient and rapid adsorption of iodide ion from aqueous solution by porous silica spheres loaded with calcined Mg-Al layered double hydroxide. J. Taiwan Inst. Chem. Eng. 2018, 85, 193–200. [Google Scholar] [CrossRef]
- Saman, N.; Othman, N.S.; Chew, L.-Y.; Mohd Setapar, S.H.; Mat, H. Cetyltrimethylammonium bromide functionalized silica nanoparticles (MSN) synthesis using a combined sol-gel and adsorption steps with enhanced adsorption performance of oxytetracycline in aqueous solution. J. Taiwan Inst. Chem. Eng. 2020, 112, 67–77. [Google Scholar] [CrossRef]
- Manyangadze, M.; Chikuruwo, N.H.M.; Narsaiah, T.B.; Chakra, C.S.; Radhakumari, M.; Danha, G. Enhancing adsorption capacity of nano-adsorbents via surface modification: A review. S. Afr. J. Chem. Eng. 2020, 31, 25–32. [Google Scholar] [CrossRef]
- Rahman, I.A.; Jafarzadeh, M.; Sipaut, C.S. Synthesis of organo-functionalized nanosilica via a co-condensation modification using γ-aminopropyltriethoxysilane (APTES). Ceram. Int. 2009, 35, 1883–1888. [Google Scholar] [CrossRef]
- Kamarudin, N.H.N.; Jalil, A.A.; Triwahyono, S.; Salleh, N.F.M.; Karim, A.H.; Mukti, R.R.; Hameed, B.H.; Ahmad, A. Role of 3-aminopropyltriethoxysilane in the preparation of mesoporous silica nanoparticles for ibuprofen delivery: Effect on physicochemical properties. Micropor. Mesopor. Mater. 2013, 180, 235–241. [Google Scholar] [CrossRef]
- Shylesh, S.; Samuel, P.P.; Sisodiya, S.; Singh, A.P. Periodic mesoporous silicas and organosilicas: An overview towards catalysis. Catal. Surv. Asia 2008, 12, 266–282. [Google Scholar] [CrossRef]
- Wang, G.; Otuonye, A.N.; Blair, E.A.; Denton, K.; Tao, Z.; Asefa, T. Functionalized mesoporous materials for adsorption and release of different drug molecules: A comparative Study. J. Solid State Chem. 2009, 182, 1649–1660. [Google Scholar] [CrossRef]
- Delacôte, C.; Gaslain, F.O.M.; Lebeau, B.; Walcarius, A. Factors affecting the reactivity of thiol-functionalized mesoporous silica adsorbents toward mercury(II). Talanta 2009, 79, 877–886. [Google Scholar] [CrossRef]
- Ritter, H.; Nieminen, M.; Karppinen, M.; Brühwiler, D. A comparative study of the functionalization of mesoporous silica MCM-41 by deposition of 3-aminopropyltrimethoxysilane from toluene and from the vapor phase. Micropor. Mesopor. Mater. 2009, 121, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.-Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruk, M.; Asefa, T.; Jaroniec, M.; Ozin, G.A. Synthesis and characterization of methyl- and vinyl-functionalized ordered mesoporous silicas with high organic content. In Nanoporous Materials III., Surface Science and Catalysis; Sayari, A., Jaroniec, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 141, pp. 197–204. [Google Scholar] [CrossRef]
- Sharifi, M.; Wallacher, D.; Wark, M. Distribution of functional groups in periodic mesoporous organosilica materials studied by small-angle neutron scattering with in situ adsorption of nitrogen. Beilstein J. Nanotechnol. 2012, 3, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putz, A.-M.; Policicchio, A.; Stelitano, S.; Sfîrloagă, P.; Ianăşi, C.; Agostino, R.G.; Cecilia, S. Tailoring mesoporous silica by functionalization for gases (H2, CH4, CO2) storage applications. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 810–819. [Google Scholar] [CrossRef]
- Putz, A.-M.; Almásy, L.; Len, A.; Ianăşi, C. Functionalized silica materials synthesized via co-condensation and post-grafting methods. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 323–332. [Google Scholar] [CrossRef]
- Policicchio, A.; Conte, G.; Stelitano, S.; Bonaventura, C.P.; Putz, A.-M.; Ianăşi, C.; Almásy, L.; Horváth, Z.E.; Agostino, R.G. Hydrogen storage performances for mesoporous silica synthesized with mixed tetraethoxysilane and methyltriethoxysilane precursors in acidic condition. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 125040. [Google Scholar] [CrossRef]
- Showkat, A.M.; Zhang, Y.P.; Min, S.K.; Gopalan, A.I.; Reddy, K.R.; Lee, K.P. Analysis of heavy metal toxic ions by adsorption onto amino-functionalized ordered mesoporous silica. Bull. Korean Chem. Soc. 2007, 28, 1985–1992. [Google Scholar] [CrossRef] [Green Version]
- Talavera-Pech, W.A.; Esparza-Ruiz, A.; Quintana-Owen, P.; Vilchis-Nestor, A.R.; Carrera-Figueiras, C.; Ávila-Ortega, A. Effects of different amounts of APTES on physicochemical and structural properties of amino-functionalized MCM-41-MSNs. J. Sol. Gel. Sci. Technol. 2016, 80, 697–708. [Google Scholar] [CrossRef]
- GracePavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P.A. Review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Malaviya, P.; Singh, A. Bioremediation of chromium solutions and chromium containing wastewaters. Crit. Rev. Microbiol. 2016, 42, 607–633. [Google Scholar] [CrossRef]
- Singh, S.; Kang, S.H.; Mulchandani, A.; Chen, W. Bioremediation: Environmental clean-up through pathway engineering. Curr. Opin. Biotechnol. 2008, 19, 437–444. [Google Scholar] [CrossRef]
- Tabinda, A.B.; Irfan, R.; Yasar, A.; Iqbal, A.; Mahmood, A. Phytoremediation potential of Pistia stratiotes and Eichhornia crassipes to remove chromium and copper. Environ. Technol. 2020, 41, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.S.; Reddy, A.S.; Kalia, R.K.; Thukral, A.K. Modeling and optimization of voltage and treatment time for electrocoagulation removal of hexavalent chromium. Desalination 2011, 269, 157–162. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Yashwanthraj, M. Sequestration of toxic Cr(VI) ions from industrial wastewater using waste biomass: A review. Desalin. Water. Treat. 2017, 68, 245–266. [Google Scholar] [CrossRef]
- Suganya, S.; Senthil Kumar, P. Influence of ultrasonic waves on preparation of active carbon from coffee waste for the reclamation of effluents containing Cr(VI) ions. J. Ind. Eng. Chem. 2018, 60, 418–430. [Google Scholar] [CrossRef]
- Latha, A.; Reddy, S. Review on bioremediation–Potential tool for removing environmental pollution. Int. J. Basic Appl. Chem. Sci. 2013, 3, 21–33. [Google Scholar]
- Sane, P.; Chaudhari, S.; Nemade, P.; Sontakke, S. Photocatalytic reduction of chromium (VI) using combustion synthesized TiO2. J. Environ. Chem. Eng. 2018, 6, 68–73. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Tuo, X.; Almásy, L.; Tian, Q.; Sun, G.; Henderson, M.J.; Li, Q.; Wacha, A.; Courtois, J.; et al. Thickness determination of ultrathin poly (acrylic acid) shell on γ-Fe2O3 nanocore via small-angle scattering. Mater. Chem. Phys. 2018, 204, 236–242. [Google Scholar] [CrossRef]
- Zhu, S.; Leng, Y.; Yan, M.; Tuo, X.; Yang, J.; Almásy, L.; Tian, Q.; Sun, G.; Zou, L.; Li, Q.; Courtois, J.; Zhang, H. Bare and polymer coated iron oxide superparamagnetic nanoparticles for effective removal of U(VI) from acidic and neutral aqueous medium. Appl. Surf. Sci. 2018, 447, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Kuklin, A.I.; Soloviov, D.V.; Rogachev, A.V.; Utrobin, P.K.; Kovalev, Y.S.; Balasoiu, M.; Ivankov, O.I.; Sirotin, A.P.; Murugova, T.N.; Petukhova, T.B.; et al. New opportunities provided by modernized small-angle neutron scattering two-detector system instrument (YuMO). J. Phys. Conf. Ser. 2011, 291, 12013. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Rogov, A.D.; Gorshkova, Y.E.; Utrobin, P.K.; Kovalev, Y.S.; Rogachev, A.V.; Ivankov, O.I.; Kutuzov, S.A.; Soloviov, D.V.; et al. Analysis of neutron spectra and fluxes obtained with cold and thermal moderators at IBR-2 reactor: Experimental and computer-modeling studies. Phys. Part. Nucl. Lett. 2011, 8, 119. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Islamov, A.K.; Gordeliy, V.I. Scientific reviews: Two-detector system for small-angle neutron scattering instrument. Neutr. News 2005, 16, 16–18. [Google Scholar] [CrossRef]
- Nyam-Osor, M.; Soloviov, D.V.; Kovalev, Y.S.; Zhigunov, A.; Rogachev, A.V.; Ivankov, O.I.; Erhan, R.V.; Kuklin, A.I. Silver behenate and silver stearate powders for calibration of SAS instruments. J. Phys. Conf. Ser. 2012, 351, 12024. [Google Scholar] [CrossRef]
- Soloviev, A.G.; Solovjeva, T.M.; Ivankov, O.I.; Soloviov, D.V.; Rogachev, A.V.; Kuklin, A.I. SAS program for two-detector system: Seamless curve from both detectors. J. Phys. Conf. Ser. 2017, 848, 012020. [Google Scholar] [CrossRef]
- Borah, D.; Satokawa, S.; Kato, S.; Kojima, T. Sorption of As(V) from aqueous solution using acid modified carbon black. J Hazard. Mater. 2009, 162, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Borah, D.; Satokawa, S.; Kato, S.; Kojima, T. Surface-modified carbon black for As(V) removal. J. Colloid. Interf. Sci. 2008, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almásy, L.; Putz, A.M.; Tian, Q.; Kopitsa, G.P.; Khamova, T.V.; Barabás, R.; Rigó, M.; Bóta, A.; Wacha, A.; Mirica, M.; et al. Hybrid mesoporous silica with controlled drug release. J. Serb. Chem. Soc. 2019, 84, 1027–1039. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R’’Si(OR’)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Maria Chong, A.S.; Zhao, X.S. Functionalization of SBA-15 with APTES and characterization of functionalized materials. J. Phys. Chem. B 2003, 107, 12650–12657. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.L.; Mustafa, N.N.N. Pore surface fractal analysis of palladium-alumina ceramic membrane using frenkel-halsey-hill (FHH) model. J. Colloid Interf. Sci. 2006, 301, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Putz, A.-M.; Cecilia, S.; Ianăşi, C.; Dudás, Z.; Székely, K.N.; Plocek, J.; Sfârloagă, P.; Săcărescu, L.; Almásy, L. Pore ordering in mesoporous matrices induced by different directing agents. J. Porous Mater. 2015, 22, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Almásy, L.; Putz, A.-M.; Len, A.; Plestil, J.; Savii, C. Small-angle scattering investigation of silica xerogels and sonogels prepared with ionic liquid pyridinium tetrafluoroborate. Process. Appl. Ceram. 2017, 11, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Putz, A.-M.; Wang, K.; Len, A.; Plocek, J.; Bezdicka, P.; Kopitsa, G.P.; Khamova, T.V.; Ianăşi, C.; Săcărescu, L.; Mitróová, Z.; et al. Mesoporous silica obtained with methyltriethoxysilane as co-precursor in alkaline medium. Appl. Surf. Sci. 2017, 424, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Osta, O.; Bombled, M.; Partouche, D.; Gallier, F.; Lubin-Germain, N.; Brodie-Linder, N.; Alba-Simionesco, C. Direct synthesis of mesoporous organosilica and proof-of-concept applications in lysozyme adsorption and supported catalysis. ACS Omega 2020, 5, 18842–18848. [Google Scholar] [CrossRef]
- Borówka, A. Effects of twin methyl groups insertion on the structure of templated mesoporous silica materials. Ceram. Int. 2019, 45, 4631–4636. [Google Scholar] [CrossRef]
- Bloemen, M.; Brullot, W.; Luong, T.T.; Geukens, N.; Gils, A.; Verbiest, T. Improved functionalization of oleic acid-coated iron oxide nanoparticles for biomedical applications. J. Nanopart. Res. 2012, 14, 1100. [Google Scholar] [CrossRef] [Green Version]
- Ciopec, M.; Negrea, A.; Davidescu, C.M.; Negrea, P.; Muntean, C.; Popa, A. Use of Di-(2-Ethylhexyl) phosphoric acid (DEHPA) impregnated XAD-8 copolymer resin for the separation of metal ions from water. Revista Chimie 2010, 55, 127–131. [Google Scholar]
- Ciopec, M.; Davidescu, C.; Negrea, A.; Muntean, C.; Popa, A.; Negrea, P.; Lupa, L. Equilibrium and kinetic studies of the adsorption of Cr(III) ions onto amberlite XAD-8 impregnated with Di-(2-Ethylhexyl) phosphoric acid (DEHPA). Adsorp. Sci. Technol. 2011, 29, 989–1005. [Google Scholar] [CrossRef]
- Padmavathy, K.S.; Madhu, G.; Haseena, P.V. A study on effects of pH, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr (VI)) from wastewater by magnetite nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Bhatt, R.; Sreedhar, B.; Padmaja, P. Chitosan supramolecularly cross linked with trimesic acid–Facile synthesis, characterization and evaluation of adsorption potential for chromium(VI). Int. J. Biol. Macromol. 2017, 104, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y. Adsorption characteristics of hexavalent chromium on HCB/TiO2. Appl. Surf. Sci. 2014, 316, 649–656. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, J.; Liu, Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J. Hazard. Mater. 2009, 172, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Justi, K.C.; Fávere, V.T.; Laranjeira, M.C.M.; Neves, A.; Peralta, R.A. Kinetics and equilibrium adsorption of Cu(II), Cd(II), and Ni(II) ions by CHITO SAN futionalized with 2[-Bis-(Pyridylmethyl)Aminomethyl]-4-Methyl-6-Formylphenol. J. Colloid Interf. Sci. 2005, 291, 369–374. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Ab Ghani, S.; Kamari, A. Adsorption behaviour of Fe(II) and Fe(III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. 2005, 96, 443–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Cheng, W.; Wang, J.; Ma, J. Adsorption equilibrium and kinetics of the removal of ammoniacal nitrogen by zeolite X/activated carbon composite synthesized from elutrilithe. J. Chem. 2017, 2017, 1936829. [Google Scholar] [CrossRef]
- Ben Khalifa, E.; Rzig, B.; Chakroun, R.; Nouagui, H.; Hamrouni, B. Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent. Chemometr. Intell. Lab. Syst. 2019, 189, 18–26. [Google Scholar] [CrossRef]
- Mutongo, F.; Kuipa, O.; Kuipa, P.K. Removal of Cr(VI) from aqueous solutions using powder of potato peelings as a low cost sorbent. Bioinorg. Chem. Appl. 2014, 2014, 973153. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Saeed, K. Decontamination of Cr(VI) and Mn(II) from aqueous media by untreated and chemically treated banana peel: A comparative study. Desalin. Water Treat. 2015, 53, 3586–3591. [Google Scholar] [CrossRef]
- Sharma, P.K.; Ayub, S.; Tripathi, C.N. Isotherms describing physical adsorption of Cr(VI) from aqueous solution using various agricultural wastes as adsorbents. Cogent Eng. 2016, 3, 1186857. [Google Scholar] [CrossRef]
- Yi, Y.; Lv, J.; Liu, Y.; Wu, G. Synthesis and application of modified litchi peel for removal of hexavalent chromium from aqueous solutions. J. Mol. Liq. 2017, 225, 28–33. [Google Scholar] [CrossRef]
- Nag, S.; Mondal, A.; Bar, N.; Das, S.K. Biosorption of chromium (VI) from aqueous solutions and ann modelling. Environ. Sci. Pollut. Res. 2017, 24, 18817–18835. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.-H.; Pack, S.P.; Kim, I.; Chung, S. A systematic study of hexavalent chromium adsorption and removal from aqueous environments using chemically functionalized amorphous and mesoporous silica nanoparticles. Sci. Rep. 2020, 10, 5558. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A.; Garud, H.B.; Thoravat, S.S.; Patil, V.S.; Shinde, P.S.; Burungale, S.H.; Patil, P.S. Synthesis and testing of functional mesoporous silica nanoparticles for removal of Cr(VI) ions from water. Biointerface Res. Appl. Chem. 2021, 11, 8599–8607. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, K.; Liu, Q.Q.; Liu, Q.Q.; Yao, J.; Huang, L.; Miao, J.; Shuai, Q.; Saman, N.; Othman, N.S.; et al. Green and simple synthesis of poly (Catechol-Tetraethylenepentamine)@aminopropyl-modified silica composite for removing toxic Cr(VI). J. Taiwan Inst. Chem. Eng. 2020, 110, 112–119. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Choi, K.; Kim, H.G.; Park, J.A.; Cho, S.H.; Hong, S.W.; Lee, J.H.; Lee, J.H.; Lee, S.; et al. Investigation of the mechanism of chromium removal in (3-aminopropyl)trimethoxysilane functionalized mesoporous silica. Sci. Rep. 2018, 8, 12078. [Google Scholar] [CrossRef]
- Li, X.; Han, C.; Zhu, W.; Ma, W.; Luo, Y.; Zhou, Y.; Yu, J.; Wei, K. Cr(VI) removal from aqueous by adsorption on amine-functionalized mesoporous silica prepared from Silica FUME. J. Chem. 2014, 2014, 765856. [Google Scholar] [CrossRef]
- Choi, K.; Lee, S.; Park, J.O.; Park, J.-A.; Cho, S.-H.; Lee, S.Y.; Lee, J.H.; Choi, J.-W. Chromium removal from aqueous solution by a pei-silica nanocomposite. Sci. Rep. 2018, 8, 1438. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Gao, P.; Liu, Y.; Minami, T.; Yu, C. Removal of Cr(VI) from aqueous solution by polypyrrole/hollow mesoporous silica particles. Nanomaterials 2020, 10, 686. [Google Scholar] [CrossRef] [Green Version]













| Sample | SBET (Specific Surface Area (Total)) (m2/g) | Micropore Area (m2/g) | dDFT (nm) | VT (Total Pore Volume) (cm3/g) | df FHH (ads/des) |
|---|---|---|---|---|---|
| A-10-co-cond | 500 | 183 | 3.6 | 0.34 | 2.13/2.71 |
| A-15-co-cond | 323 | 145 | 3.5 | 0.22 | 2.18/2.72 |
| A-10-PG | 416 | 289 | 3.2 | 0.23 | 2.18/2.72 |
| A-15-PG | 93 | 37 | 5.3 | 0.11 | 1.90/2.63 |
| T, K | qe,exp, mg/g | Pseudo-First Order Kinetic Model | Pseudo-Second Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| qe,calc, mg/g | k1, min−1 | R2 | qe,calc, mg/g | k2, min−1(mg/g)−1 | R2 | ||
| A-10-co-cond | |||||||
| 298 | 22.4 | 15.8 | 0.0132 | 0.8849 | 26.3 | 128.9 | 0.9994 |
| 308 | 23.4 | 13.7 | 0.0162 | 0.8975 | 26.4 | 625.6 | 0.9982 |
| 318 | 24.3 | 13.5 | 0.0237 | 0.9588 | 27.1 | 910.6 | 0.9926 |
| A-10-PG | |||||||
| 298 | 3.7 | 1.2 | 0.008 | 0.9355 | 5.7 | 2.77 | 0.9974 |
| 308 | 4.5 | 1.8 | 0.013 | 0.9145 | 5.8 | 4.33 | 0.9913 |
| 318 | 4.7 | 2.2 | 0.016 | 0.918 | 5.2 | 6.78 | 0.9998 |
| Materials | Temperture, K | ΔG0, kJ/mol | ΔH0, kJ/mol | ΔS0, kJ/mol∙K |
|---|---|---|---|---|
| A-10-co-cond | 298 | −1.9 | 42.1 | 0.14 |
| 308 | −3.4 | |||
| 318 | −4.8 | |||
| A-10-PG | 298 | −1.6 | 42.9 | 0.15 |
| 308 | −3.1 | |||
| 318 | −4.6 |
| Freundlich Isotherm | Langmuir Isotherm | ||||
|---|---|---|---|---|---|
| KF, mg/g | 1/n | R2 | KL, L/mg | qm calc, mg/g | R2 |
| A-10-co-cond | |||||
| 13.2 | 0.31 | 0.9848 | 0.02 | 93.6 | 0.9969 |
| A-10-PG | |||||
| 2.01 | 0.36 | 0.9749 | 0.06 | 11.5 | 0.9949 |
| Adsorbent and Morphology | Adsorption Capacity, mg/g | Specific Surface Area (SBET) and Total Pore Volume (VT) | Reference |
|---|---|---|---|
| Orange peel | 7.14 | - | [58] |
| Potato peel | 3.28 | - | [59] |
| Banana peel | 3.35 | - | [60] |
| Pea pod | 4.33 | - | [61] |
| Litchi peel | 7.05 | - | [62] |
| Coconut shell | 8.73 | SBET = 1.49 m2/g; VT = 0.683 cm3/g | [63] |
| Garlic peel | 9.22 | SBET = 3.21 m2/g; VT = 0.191 cm3/g | [63] |
| Amorphous silica nanoparticle | 0.4 | SBET = 387 m2/g; VT = 0.66 cm3/g | [64] |
| Ordered mesoporous silica nanoparticle | 1.3 | SBET = 792.1 m2/g; VT = 2.11 cm3/g | [64] |
| amino-functionalized amorphous silica nanoparticle | 33.9 | SBET = 189.9 m2/g; VT = 0.60 cm3/g | [64] |
| Amino-functionalized mesoporous silica nanoparticle | 42.1 | SBET = 213.5 m2/g; VT = 1.42 cm3/g | [64] |
| Amino functionalized mesoporous silica (hexagonal pore structure) | 50 | SBET = 721 m2/g; | [65] |
| Poly(catechol-tetraethylene-pent-amine) @SiO2-NH2 | 400.8 | - | [66] |
| Aminopropyl functionalized mesoporous silica by post-grafting (Primary particles of 50–60 nm in size formed agglomerates of 700–800 nm in size) | 39.3;82.5; 84.3 | SBET = 1379.6 m2/g; VT = 1.7 cm3/g SBET = 857.88 m2/g; VT = 0.0698 cm3/g SBET = 682.06 m2/g; VT = 0.256 cm3/g | [67] |
| Amine functionalized mesoporous silica with well-ordered hexagonal pore structure | 47.27 | - | [68] |
| Polyethylenimine-silica nanocomposite Particles with near spherical and elliptical morphologies, with size in range 200–300 nm, uniformly distributed Si and N atoms | 183.7 | SBET = 21.6 m2/g; VT = 0.07 cm3/g | [69] |
| Polypyrrole/hollow mesoporous silica particle (energy-dispersive X-ray spectroscopy analysis showed the uniform distribution of N element of pyrrole) | 382 | - | [70] |
| A-10-co-cond | 85.4 | SBET = 500 m2/g; VT = 0.34 cm3/g | This paper |
| A-10-PG | 9.4 | SBET = 416 m2/g; VT = 0.23 cm3/g | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putz, A.-M.; Ciopec, M.; Negrea, A.; Grad, O.; Ianăşi, C.; Ivankov, O.I.; Milanović, M.; Stijepović, I.; Almásy, L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials 2021, 14, 628. https://doi.org/10.3390/ma14030628
Putz A-M, Ciopec M, Negrea A, Grad O, Ianăşi C, Ivankov OI, Milanović M, Stijepović I, Almásy L. Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials. 2021; 14(3):628. https://doi.org/10.3390/ma14030628
Chicago/Turabian StylePutz, Ana-Maria, Mihaela Ciopec, Adina Negrea, Oana Grad, Cătălin Ianăşi, Oleksandr I. Ivankov, Marija Milanović, Ivan Stijepović, and László Almásy. 2021. "Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods" Materials 14, no. 3: 628. https://doi.org/10.3390/ma14030628
APA StylePutz, A. -M., Ciopec, M., Negrea, A., Grad, O., Ianăşi, C., Ivankov, O. I., Milanović, M., Stijepović, I., & Almásy, L. (2021). Comparison of Structure and Adsorption Properties of Mesoporous Silica Functionalized with Aminopropyl Groups by the Co-Condensation and the Post Grafting Methods. Materials, 14(3), 628. https://doi.org/10.3390/ma14030628