Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Alkali-Sodium Surface Treatment of Samples
2.3. Apatite-Forming Ability
2.4. Surface Characterization
2.5. Contact Angle
2.6. Cell Lines
2.7. Cell Viability
2.8. Cellular Morphology
2.9. Statistical Analysis
3. Results
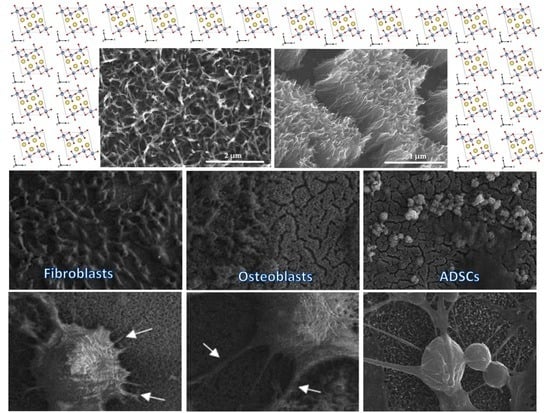
3.1. Surface Characterization
3.2. Apatite-Forming Ability
3.3. Cell Viability Measured with the MTT Assay
3.4. Cell Morphology Observed by Scanning Electron Microscopy
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piszczek, P.; Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sznarkowska, A.; Sadowska, B.; Bartmański, M.; Erdogan, Y.K.; Ercan, B.; Jędrzejczyk, W. Comprehensive Evaluation of the Biological Properties of Surface-Modified Titanium Alloy Implants. J. Clin. Med. 2020, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Ehlert, M.; Roszek, K.; Jędrzejewski, T.; Bartmański, M.; Radtke, A. Titania Nanofiber Scaffolds with Enhanced Biointegration Activity—Preliminary In Vitro Studies. Int. J. Mol. Sci. 2019, 20, 5642. [Google Scholar] [CrossRef] [Green Version]
- Ehlert, M.; Radtke, A.; Jędrzejewski, T.; Roszek, K.; Bartmański, M.; Piszczek, P. In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells. Materials 2020, 13, 1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Biomaterials and Tissue Biomechanics: A Match Made in Heaven? Materials 2017, 10, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, M.; Hooper, G.; Frampton, C.; Rothwell, A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J. Orthop. 2014, 5, 591–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.C.; Dunand, D.C. Mechanical properties of directionally freeze-cast titanium foams. Acta Mater. 2011, 59, 146–158. [Google Scholar] [CrossRef]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.; Su, B.; Dalby, M.J. Osteogenic and bactericidal surfaces from hydrothermal titania nanowires on titanium substrates. Sci. Rep. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Lah, N.A.C.; Hussin, M.H. Titanium and Titanium Based Alloys as Metallic Biomaterials in Medical Applications—Spine Implant Case Study. Pertanika J. Sci. Technol. 2019, 27, 459–472. [Google Scholar]
- Soro, N.; Attar, H.; Brodie, E.; Veidt, M.; Molotnikov, A.; Dargusch, M.S. Evaluation of the mechanical compatibility of additively manufactured porous Ti–25Ta alloy for load-bearing implant applications. J. Mech. Behav. Biomed. Mater. 2019, 97, 149–158. [Google Scholar] [CrossRef]
- Verma, R.P. Titanium based biomaterial for bone implants: A mini review. Mater. Today Proc. 2020, 26, 2214–7853. [Google Scholar] [CrossRef]
- Trujillo, C.D.; Beltran, A.M.; Garvi, D.; Salazar-Moya, A.; Lebrato-Martinez, J.; Hickey, D.J.; Rodriguez-Ortiz, J.A.; Kamm, P.H.L.; Lebrato, C.; Garcia-Moreno, F.; et al. Bacterial behavior on coated porous titanium substrates for biomedical applications. Surf. Coat. Technol. 2019, 357, 896–902. [Google Scholar] [CrossRef]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—Is one truly better than the other? Mater. Sci Eng. C 2016, 62, 960–966. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Leal, J.I.; Rodriguez-Valverde, M.A.; Mazzaglia, G.; Ramon-Torregrosa, P.J.; Rodriguez, L.D.; Garcia-Martinez, O.; Vallecillo-Capilla, M.; Ruiz, C.; Cabrerizo-Vilchez, M.A. Effect of roughness, wettability and morphology of engineered titanium surfaces on osteoblast-like cell adhesion. Colloids Surf. A Physicochem. Eng. Aspects 2010, 365, 222–229. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Guo, X.; Hu, J. Surface morphology of modified titanium alloy affects proliferation stability of bone marrow mesenchymal stem cells. Surf. Coat. Technol. 2019, 366, 156–163. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Ponche, A.; Bigerelle, M.; Anselme, K. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 1: Physico-chemical effects. Proc. Inst. Mech. Eng. 2010, 224, 1471–1486. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast– biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Anselme, K.; Ponche, A.; Bigerelle, M. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. Proc. Inst. Mech. Eng. 2010, 224, 1487–1507. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Bartmański, M. The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. J. Clin. Med. 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radtke, A.; Bal, M.; Jędrzejewski, T. Novel Titania Nanocoatings Produced by Anodic Oxidation with the Use of Cyclically Changing Potential: Their Photocatalytic Activity and Biocompatibility. Nanomaterials 2018, 8, 712. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Qian, Q.; Xu, L.; Zhu, H.; Xu, L.; Wang, Q. Effects of hydrothermal sterilization on properties of biological coating fabricated by alkaline-heat treatment on titanium. Surf. Coat. Technol. 2018, 342, 69–75. [Google Scholar] [CrossRef]
- Wang, H.; Lai, Y.; Zheng, R.; Bian, Y.; Zhang, K.; Lin, C. Tuning the surface microstructure of titanate coatings on titanium implants for enhancing bioactivity of implants. Int. J. Nanomed. 2015, 10, 3887–3896. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.K.; Hussein, M.A.; Adesina, A.Y.; Al-Aqeeli, N. In Vitro Corrosion and Bioactivity Performance of Surface-Treated Ti-20Nb-13Zr Alloys for Orthopedic Applications. Coatings 2019, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Kim, Y.D.; Shin, J.H.; Lee, K.H. Surface modification by alkali and heat treatments intitanium alloys. J. Biomed. Mater. Res. 2002, 61, 466–473. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Rakngarm, A.; Miyashita, Y.; Mutoh, Y. Formation of hydroxyapatite layer on bioactive Ti and Ti–6Al–4V by simple chemical technique. J. Mater. Sci. Mater. Med. 2008, 19, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- He, D.H.; Wang, P.; Liu, P.; Liu, X.K.; Ma, F.C.; Zhao, J. HA coating fabricated by electrochemical deposition on modified Ti6Al4V alloy. Surf. Coat. Technol. 2016, 301, 6–12. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Zhou, Z.; Shen, L.; Bao, N. Microstructure and Morphology Control of Potassium Magnesium Titanates and Sodium Iron Titanates by Molten Salt Synthesis. Materials 2019, 12, 1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, S.; Korosi, L.; Meynen, V.; Cool, P.; Vansant, E.F.; Dekany, I. The influence of temperature on the structural behaviour of sodium tri- and hexa-titanates and their protonated forms. J. Solid State Chem. 2005, 178, 1614–1619. [Google Scholar] [CrossRef]
- Becker, I.; Hofmann, I.; Muller, F.A. Preparation of bioactive sodium titanate ceramics. J. Eur. Ceram. Soc. 2007, 27, 4547–4553. [Google Scholar] [CrossRef]
- Sauvet, A.L.; Baliteau, S.; Lopez, C. Synthesis and characterization of sodium titanates Na2Ti3O7 and Na2Ti6O13. J. Solid State Chem. 2004, 177, 4508–4515. [Google Scholar] [CrossRef]
- Wadsley, A.D.; Mumme, W.G. The Crystal Structure of Na2Ti7O15, and an ordered intergrowth of Na2Ti6O13 and ‘Na2Ti8O17′. Acta Cryst. 1968, B24, 392–396. [Google Scholar] [CrossRef]
- Wei, M.; Kim, H.M.; Kokubo, T.; Evans, J.H. Optimising the bioactivity of alkaline-treated titanium alloy. Mater. Sci. Eng. C 2002, 20, 125–134. [Google Scholar] [CrossRef]
- Do Nascimento, R.M.; de Carvalho, V.R.; Govone, J.S.; Hernandes, A.C.; da Cruz, N.C. Effects of negatively and positively charged Ti metal surfaces on ceramic coating adhesion and cell respons. J. Mater. Sci. Mater. Med. 2017, 28, 33. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Yamaguchi, S. Novel Bioactive Titanate Layers Formed on Ti Metal and Its Alloys by Chemical Treatments. Materials 2010, 3, 48–63. [Google Scholar] [CrossRef]
- Baliteau, S.; Sauvet, A.L.; Lopez, C.; Fabry, P. Controlled synthesis and characterization of sodium titanate composites Na2Ti3O7/Na2Ti6O13. Solid State Ionics 2007, 178, 1517–1522. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvag, H.; Norby, P. Crystal Structures of Titanate Nanotubes: A Raman Scattering Study. Inorg. Chem. 2009, 48, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, K.; Ise, K.; Goto, K.; Akiyama, H.; Nakamura, T.; Kaneuji, A.; Sugimori, T.; Matsumoto, T. A New Cementless Total Hip Arthroplasty With Bioactive Titanium Porous-Coating by Alkaline and Heat Treatment: Average 4.8-Year Results. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, X.; He, D.; Liu, P.; Ma, F.; Li, Q.; Feng, N. Influence of Alkali Treatment on Ti6Al4V Alloy and the HA Coating Deposited by Hydrothermal-Electrochemical Methods. Rare Metal. Mater. Eng. 2014, 43, 830–835. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Effect of heat treatment on apatite-forming ability of Ti metal induced by alkali treatment. J. Mater. Sci. Mater. Med. 1997, 8, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Le, P.T.M.; Ito, M.; Shintani, S.A.; Takadama, H. Tri-Functional Calcium-Deficient Calcium Titanate Coating on Titanium Metal by Chemical and Heat Treatment. Coatings 2019, 9, 561. [Google Scholar] [CrossRef] [Green Version]
- Takadama, H.; Kim, H.M.; Kokubo, T.; Nakamura, T. An X-ray photoelectron spectroscopy study of the process of apatite formation on bioactive titanium metal. J. Biomed. Mater. Res. 2001, 55, 185–193. [Google Scholar] [CrossRef]
- Obata, A.; Zhai, T.; Kasuga, T. Apatite-forming ability on titanium surface modified by hydrothermal treatment and ultraviolet irradiation. J. Mater. Res. 2008, 23, 3169–3175. [Google Scholar] [CrossRef]
- Koju, N.; Sikder, P.; Ren, Y.; Zhou, H.; Bhaduri, S.B. Biomimetic coating technology for orthopedic implants. Curr. Opin. Chem. Eng. 2017, 15, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Effect of carbonate content and buffer type on calcium phosphate formation in SBF solutions. J. Mater. Sci. Mater. Med. 2006, 17, 697–707. [Google Scholar] [CrossRef]
- Fatehi, K.; Moztarzadeh, F.; Solati-Hashjin, M.; Tahriri, M.; Rezvannia, M.; Ravarian, R. In vitro biomimetic deposition of apatite on alkaline and heat treated Ti6Al4V alloy surface. Bull. Mater. Sci. 2008, 31, 101–108. [Google Scholar] [CrossRef]
- Jonasova, L.; Muller, F.A.; Helebrant, A.; Strnad, J.; Greil, P. Biomimetic apatite formation on chemically treated titanium. Biomaterials 2004, 25, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Jun, Y.K.; Han, Y.; Hong, S.H. Biomimetic Apatite Coatings on Micro-Arc Oxidized Titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef]
- ISO/FDIS 23317:2007(E) Standards. Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. Available online: https://www.iso.org/standard/41446.html (accessed on 5 March 2019).
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Manfroi, D.C.; dos Anjos, A.; Cavalheiro, A.A.; Perazolli, L.A.; Varela, J.A.; Zaghete, M.A. Titanate nanotubes produced from microwave-assisted hydrothermal synthesis: Photocatalytic and structural properties. Ceram. Int. 2014, 40, 14483–14491. [Google Scholar] [CrossRef]
- Morgan, D.L.; Zhu, H.Y.; Frost, R.L.; Waclawik, E.R. Determination of a Morphological Phase Diagramof Titania/Titanate Nanostructures from AlkalineHydrothermal Treatment of Degussa P25. Chem. Mater. 2008, 20, 3800–3802. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Yang, S. Three-dimensional spider-web architecture assembled from Na2Ti3O7nanotubes as a high performance anode for a sodium-ion battery. Chem. Commun. 2014, 50, 14029. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Jiang, H.; Liu, T.; Liu, X.; Meng, G. Preparation and photocatalytic activity of alkali titanate nano materials A2TinO2n+1(A = Li, Na and K). Mater. Res. Bull. 2007, 42, 334–344. [Google Scholar] [CrossRef]
- Zhang, Z.; Goodall, J.B.; Brown, S.; Karlsson, L.; Clark, R.J.; Hutchison, J.L.; Rehman, I.U.; Darr, J.A. Continuous hydrothermal synthesis of extensive 2D sodium titanate (Na2Ti3O7) nano-sheets. Dalton Trans. 2010, 39, 711–714. [Google Scholar] [CrossRef]
- Guo, Y.; Lee, N.H.; Oh, H.J.; Yoon, C.R.; Park, K.S.; Lee, H.G.; Lee, K.S.; Kim, S.J. Structure-tunable synthesis of titanate nanotube thin films via a simple hydrothermal process. Nanotechnology 2007, 18, 295608. [Google Scholar] [CrossRef]
- Yan, X.; Sun, D.; Jiang, J.; Yan, W.; Jin, Y. Self-assembled twine-like Na2Ti3O7 nanostructure as advanced anode for sodium-ion batteries. J. Alloys Compd. 2017, 697, 208–214. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Kim, D.H.; Jho, J.H.; Lee, W.J.; Lee, H.G.; Kim, S.J. Effect of post treatments on the structure and thermal stability of titanate nanotubes. Nanotechnology 2006, 17, 5922. [Google Scholar] [CrossRef]
- Bamberger, C.E.; Begun, G.M. Sodium Titanates: Stoichiometry and Raman Spectra. J. Am. Ceram. Soc. 1987, 70, 48–51. [Google Scholar] [CrossRef]
- Yin, J.; Qi, L.; Wang, H. Sodium Titanate Nanotubes as Negative Electrode Materials for Sodium-Ion Capacitors. ACS Appl. Mater. Interfaces 2012, 4, 2762–2768. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.L.; Zu-Liang, D.; Yan-Mei, M.; Xue-Fei, L.; Qi-Liang, C.; Tian, C.; Bing-Bing, L.; Guang-Tian, Z. Raman Investigation of Sodium Titanate Nanotubes under Hydrostatic Pressures up to 26.9 GPa. Chin. Phys. Lett. 2010, 27, 026103. [Google Scholar]
- Weng, L.O.; Song, S.H.; Hodgson, S.; Baker, A.; Yu, J. Synthesis and characterization of nanotubular titanates and titania. J. Eur. Ceram. Soc. 2006, 26, 1405–1409. [Google Scholar] [CrossRef]
- Ramesh, S.; Loo, Z.Z.; Tan, C.Y.; Chew, W.J.K.; Ching, Y.C.; Tarlochan, F.; Chandran, H.; Krishnasamy, S.; Bang, L.T.L.; Sarhan, A.A.D. Characterization of biogenic hydroxyapatite derived from animal bones for biomedical applications. Ceram. Int. 2018, 44, 10525–10530. [Google Scholar] [CrossRef]
- Xiao, X.F.; Liu, R.F.; Zheng, Y.Z. Characterization of hydroxyapatite/titania composite coatings codeposited by a hydrothermal–electrochemical method on titanium. Surf. Coat. Technol. 2006, 200, 4406–4413. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, H.; Qiao, H.; Zhang, X.; Jia, T.; Han, S.; Gao, Y.; Xiao, H.; Yang, H. Fabrication of silver- and strontium-doped hydroxyapatite/TiO2 nanotube bilayer coatings for enhancing bactericidal effect and osteoinductivity. Ceram. Int. 2017, 43, 992–1007. [Google Scholar] [CrossRef]
- Brangule, A.; Gross, K.A. Importance of FTIR Spectra Deconvolution for the Analysis of Amorphous Calcium Phosphates. IOP Conf. Ser. Mater. Sci. Eng. 2015, 77, 012027. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, H.; Macak, J.M.; Müller, L.; Kunze, J.; Müller, F.; Greil, P.; Virtanen, S.; Schmuki, P. Hydroxyapatite growth on anodic TiO2 nanotubes. J. Biomed. Mater. Res. A. 2006, 77, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Manso, M.; Langlet, M.; Martinez-Duart, J.M. Testing sol–gel CaTiO3 coatings for biocompatible applications. Mater. Sci. Eng. C 2003, 23, 447–450. [Google Scholar] [CrossRef]
- Barinov, S.M.; Rau, J.V.; Cesaro, S.N.; Durisin, J.; Fadeeva, I.V.; Ferro, D.; Medvecky, L.; Trionfetti, G. Carbonate release from carbonated hydroxyapatite in the wide temperature rage. J. Mater. Sci. Mater. Med. 2006, 17, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR Spectroscopy. J. Mater. Sci Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y.; Jia, D.; Wang, Y. Biomimetic apatite deposited on microarc oxidized anatase-based ceramic coating. Ceram. Int. 2008, 34, 1139–1144. [Google Scholar] [CrossRef]
- Ramesh, S.T.; Rameshbabu, N.; Gandhimathi, R.; Nidheesh, P.V.; Kumar, M.S. Kinetics and equilibrium studies for the removal of heavy metals in both single and binary systems using hydroxyapatite. Appl. Water Sci. 2012, 2, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; IntechOpen Ltd.: London, UK, 2012; Volume 6, pp. 123–148. [Google Scholar]
- Tripathy, A.; Sharma, P.; Sahoo, N.; Pramanik, S.; Osman, N.A.A. Moisture sensitive inimitable Armalcolite/PDMS flexible sensor: A new entry. Sens. Actuators B Chem. 2018, 262, 211–220. [Google Scholar] [CrossRef]
- Bohre, A.; Avasthi, K.; Singh, B.; Shrivastava, O.P. Crystallographic Evaluation of Titanate Ceramics as a Host Structure for Immobilization of Samarium. Radiochemistry 2014, 56, 92–97. [Google Scholar] [CrossRef]
- Rincon-Lopez, J.A.; Hermann-Munoz, J.A.; Giraldo-Betancur, A.L.; de Vizcaya-Ruiz, A.; Alvarado-Orozco, J.M.; Munoz-Saldana, J. Synthesis, Characterization and In Vitro Study of Synthetic and Bovine-Derived Hydroxyapatite Ceramics: A Comparison. Materials 2018, 11, 333. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, H.; Riley, C.; Coble, H.D. Analysis of apatite deposits on substrates. J. Biomed. Mater. Res. 1993, 27, 477–482. [Google Scholar] [CrossRef]
- Huong, D.T.M.; Nam, N.H.; Vu, L.V.; Long, N.N. Preparation and optical characterization of Eu3+-doped CaTiO3 perovskite powders. J. Alloys Compd. 2012, 537, 54–59. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Marques, V.S.; Sczancoski, J.C.; Escote, M.T.; Joya, M.R.; Varela, J.A.; Santos, M.R.M.C.; Pizani, P.S.; Longo, E. Synthesis, structural refinement and optical behavior of CaTiO3 powders: A comparative study of processing in different furnaces. Chem. Eng. J. 2008, 143, 299–307. [Google Scholar] [CrossRef]
- Muller, L.; Muller, F.A. Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Gajović, A.; Friscić, I.; Plodinec, M.; Iveković, D. High temperature Raman spectroscopy of titanate nanotubes. J. Mol. Struct. 2009, 924–926, 183–191. [Google Scholar] [CrossRef]
- Hernandez-Hipolito, P.; Juarez-Flores, N.; Martinez-Klimova, E.; Gomez-Cortes, A.; Bokhimi, X.; Escobar-Alarcon, L.; Klimova, T.E. Novel heterogeneous basic catalysts for biodiesel production: Sodium titanate nanotubes doped with potassium. Catal. Today 2015, 250, 187–196. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.M.; Wu, S.Y. Preparation, properties and characterization of CaTiO3-modified Pb(Fe1/2Nb1/2)O3 dielectrics. J. Eur. Ceram. Soc. 2003, 23, 1919–1924. [Google Scholar]
- Rahimipour, S.; Salahinejad, E.; Sharifi, E.; Nosrati, H.; Tayebi, L. Structure, wettability, corrosion and biocompatibility of nitinol treated by alkaline hydrothermal and hydrophobic functionalization for cardiovascular applications. Appl. Surf. Sci. 2020, 506, 144657. [Google Scholar] [CrossRef]
- Krząkała, A.; Służalska, K.; Dercz, G.; Maciej, A.; Kazek, A.; Szade, J.; Simka, W. Characterisation of bioactive films on Ti–6Al–4V alloy. Electrochin. Acta 2013, 104, 425–438. [Google Scholar]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Leśniak, K.; Zhidkov, I.S.; Korotin, D.M.; Kukharenko, A.I.; Cholakh, S.O.; Kalemba-Rec, I.; Suchanek, K.; Kurmaev, E.Z.; Simka, W. Influence of Alkali Treatment on Anodized Titanium Alloys in Wollastonite Suspension. Metals 2017, 7, 322. [Google Scholar] [CrossRef] [Green Version]
- Ricci, J.L.; Grew, J.C.; Alexander, H. Connective-tissue responses to defined biomaterial surfaces. I. Growth of rat fibroblast and bone marrow cell colonies on microgrooved substrates. J. Biomed. Mater. Res. A 2007, 85, 313–325. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef]
- Vannozzi, L.; Gouveia, P.; Pingue, P.; Canale, C.; Ricotti, L. Novel Ultrathin Films Based on a Blend of PEG-b-PCL and PLLA and Doped with ZnO Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21398–21410. [Google Scholar] [CrossRef]
- Strickstrock, M.; Rothe, H.; Grohmann, S.; Hildebrand, G.; Zylla, I.M.; Liefeith, K. Influence of surface roughness of dental zirconia implants on their mechanical stability, cell behavior and osseointegration. BioNanoMaterials 2017, 18, 20160013. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaswamy, Y.; Roohani, I.; No, Y.J.; Madafiglio, G.; Chang, F.; Zhang, F.; Lu, Z.; Zreiqat, H. Nature-inspired topographies on hydroxyapatite surfaces regulate stem cells behaviour. Bioact. Mater. 2020, 6, 1107–1117. [Google Scholar] [CrossRef] [PubMed]















| Functional Groups | Frequencies (Experimental) (cm−1) | Frequencies (Reference) (cm−1) | Reference |
|---|---|---|---|
| ν (Na–O–Ti) | 278 | 280 | [61,62,63] |
| δ (Ti–O) | 445 | 440, 448 | [43,63] |
| ν (Na–O–Ti) | 669 | 660 | [32,63] |
| δ (Ti–O) | 704 | 690, 700 | [43,63] |
| ν (Ti–O–Na) | 903 | 905, 920 | [43,61,63] |
| Biomaterial Sample | Average Contact Angle (°) ± Standard Deviation | |
|---|---|---|
| Measuring Liquid | ||
| Water | Diiodomethane | |
| T | 81.3 ± 0.2 | 49.2 ± 0.9 |
| T5 | 94.4 ± 0.4 | 22.4 ± 1.0 |
| T-S | ˂10 | ˂10 |
| T5-S | ˂10 | ˂10 |
| Biomaterial Sample | Ca/P (Molar Ratio) after 7 Days | Ca/P (Molar Ratio) after 14 Days | Ca/P (Molar Ratio) after 21 Days | Ca/P (Molar Ratio) after 28 Days |
|---|---|---|---|---|
| T | No growth | No growth | No growth | No growth |
| T5 | No growth | No growth | No growth | No growth |
| T-S | 7.26 | 1.94 | 2.00 | 1.82 |
| T5-S | 8.98 | 1.76 | 1.84 | 1.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlert, M.; Radtke, A.; Roszek, K.; Jędrzejewski, T.; Piszczek, P. Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties. Materials 2021, 14, 806. https://doi.org/10.3390/ma14040806
Ehlert M, Radtke A, Roszek K, Jędrzejewski T, Piszczek P. Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties. Materials. 2021; 14(4):806. https://doi.org/10.3390/ma14040806
Chicago/Turabian StyleEhlert, Michalina, Aleksandra Radtke, Katarzyna Roszek, Tomasz Jędrzejewski, and Piotr Piszczek. 2021. "Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties" Materials 14, no. 4: 806. https://doi.org/10.3390/ma14040806
APA StyleEhlert, M., Radtke, A., Roszek, K., Jędrzejewski, T., & Piszczek, P. (2021). Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties. Materials, 14(4), 806. https://doi.org/10.3390/ma14040806