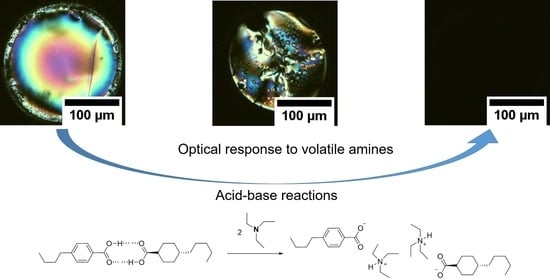
Design of Chemoresponsive Soft Matter Using Hydrogen-Bonded Liquid Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Representative Procedure Used to Synthesize the H-Bonded Liquid Crystal Mixture
2.3. Preparation of LC Thin Films on Glass Substrates
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Characterization of LC Phase Behavior
2.6. Differential Scanning Calorimetry (DSC)
2.7. Measurement of Optical Retardance
2.8. Phase Transitions Triggered by TEA
2.9. Computational Methods
3. Results and Discussion
3.1. Characterization of Hydrogen-Bonded LCs with Carboxylic Acid Groups
3.2. Experimental Characterization of Influence of TEA on the Hydrogen-Bonded LCs
3.3. Selectivity and Reversibility
3.4. Response to Complex Mixtures of Amines from Rotting Fish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mirvakili, S.M.; Hunter, I.W. Artificial Muscles: Mechanisms, Applications, and Challenges. Adv. Mater. 2018, 30, 1–28. [Google Scholar] [CrossRef]
- Zhang, L.; Naumov, P.; Du, X.; Hu, Z.; Wang, J. Vapomechanically Responsive Motion of Microchannel-Programmed Actuators. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Bonanno, L.M.; Delouise, U.A. Integration of a chemical-responsive hydrogel into a porous silicon photonic sensor for visual colorimetric readout. Adv. Funct. Mater. 2010, 20, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Pacheco, K.; Bastiaansen, C.W.M.; Broer, D.J.; Sijbesma, R.P. Optical monitoring of gases with cholesteric liquid crystals. J. Am. Chem. Soc. 2010, 132, 2961–2967. [Google Scholar] [CrossRef] [PubMed]
- Caldorera-Moore, M.E.; Liechty, W.B.; Peppas, N.A. Responsive theranostic systems: Integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Acc. Chem. Res. 2011, 44, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Kamal, T.; Park, S.-Y. A liquid crystal polymer based single layer chemo-responsive actuator. Chem. Commun. 2014, 50, 2030–2033. [Google Scholar] [CrossRef]
- Collings, P.J.; Hird, M.; Huang, C.C. Introduction to Liquid Crystals: Chemistry and Physics. Am. J. Phys. 1998, 66, 551. [Google Scholar] [CrossRef]
- Boothby, J.M.; Kim, H.; Ware, T.H. Shape changes in chemoresponsive liquid crystal elastomers. Sens. Actuators B Chem. 2017, 240, 511–518. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Onck, P.R.; Broer, D.J. Reverse switching of surface roughness in a self-organized polydomain liquid crystal coating. Proc. Natl. Acad. Sci. USA 2015, 112, 3880–3885. [Google Scholar] [CrossRef] [Green Version]
- Mezzenga, R.; Meyer, C.; Servais, C.; Romoscanu, A.I.; Sagalowicz, L.; Hayward, R.C. Shear rheology of lyotropic liquid crystals: A case study. Langmuir 2005, 21, 3322–3333. [Google Scholar] [CrossRef] [PubMed]
- Stumpel, J.E.; Wouters, C.; Herzer, N.; Ziegler, J.; Broer, D.J.; Bastiaansen, C.W.M.; Schenning, A.P.H.J. An Optical Sensor for Volatile Amines Based on an Inkjet-Printed, Hydrogen-Bonded, Cholesteric Liquid Crystalline Film. Adv. Opt. Mater. 2014, 2, 459–464. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chemie Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef] [PubMed]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and biological sensing using liquid crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef]
- Park, J.S.; Jang, C.H.; Tingey, M.L.; Lowe, A.M.; Abbott, N.L. Influence of 4-cyano-4′-biphenylcarboxylic acid on the orientational ordering of cyanobiphenyl liquid crystals at chemically functionalized surfaces. J. Colloid Interface Sci. 2006, 304, 459–473. [Google Scholar] [CrossRef]
- Yang, K.L.; Cadwell, K.; Abbott, N.L. Mechanistic study of the anchoring behavior of liquid crystals supported on metal salts and their orientational responses to dimethyl methylphosphonate. J. Phys. Chem. B 2004, 108, 20180–20186. [Google Scholar] [CrossRef]
- Yu, H.; Szilvási, T.; Rai, P.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Computational Chemistry-Guided Design of Selective Chemoresponsive Liquid Crystals Using Pyridine and Pyrimidine Functional Groups. Adv. Funct. Mater. 2018, 28, 1703581. [Google Scholar] [CrossRef]
- Yu, H.; Szilvási, T.; Wang, K.; Gold, J.I.; Bao, N.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Amplification of Elementary Surface Reaction Steps on Transition Metal Surfaces Using Liquid Crystals: Dissociative Adsorption and Dehydrogenation. J. Am. Chem. Soc. 2019, 141, 16003–16013. [Google Scholar] [CrossRef]
- Roling, L.T.; Scaranto, J.; Herron, J.A.; Yu, H.; Choi, S.; Abbott, N.L.; Mavrikakis, M. Towards first-principles molecular design of liquid crystal-based chemoresponsive systems. Nat. Commun. 2016, 7, 13338. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Abbott, N.L. Principles for Measurement of Chemical Exposure Based on Recognition-Driven Anchoring Transitions in Liquid Crystals. Science 2001, 293, 1296–1299. [Google Scholar] [CrossRef] [Green Version]
- Szilvási, T.; Bao, N.; Nayani, K.; Yu, H.; Rai, P.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Redox-Triggered Orientational Responses of Liquid Crystals to Chlorine Gas. Angew. Chemie Int. Ed. 2018, 57, 9665–9669. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.D.; Chávez, F.V.; Maria, T.M.R.; Eusebio, M.E.S.; Sebastião, P.J.; Silva, M.R. Self-assembled liquid crystals by hydrogen bonding between bipyridyl and alkylbenzoic acids: Solvent-free synthesis by mechanochemistry. Liq. Cryst. 2014, 41, 1743–1751. [Google Scholar] [CrossRef]
- Subhapriya, P.; Vijayanand, P.S.; Madhu Mohan, M.L.N. Synthesis and Characterization of Supramolecular Hydrogen-Bonded Liquid Crystals Comprising of p-n-Alkyloxy Benzoic Acids with Suberic Acid and Pimelic Acid. Mol. Cryst. Liq. Cryst. 2013, 571, 40–56. [Google Scholar] [CrossRef]
- Pongali Sathya Prabu, N.; Madhu Mohan, M.L.N. Characterization of a new smectic ordering in supramolecular hydrogen bonded liquid crystals by X-ray, optical and dielectric studies. J. Mol. Liq. 2013, 182, 79–90. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Thermotropic Liquid Crystals Formed by Intermolecular Hydrogen Bonding Interactions. Angew. Chemie Int. Ed. English 1995, 34, 1696–1711. [Google Scholar] [CrossRef]
- Bruce, D.W. Liquid Crystals Formed from Specific Supramolecular Interactions. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- He, W.; Pan, G.; Yang, Z.; Zhao, D.; Niu, C.; Huang, W.; Yuan, X.; Cuo, J.; Cao, H.; Yang, H. Wide blue phase range in a hydrogen-bonded self-assembled complex of chiral fluoro-substituted benzoic acid and pyridine derivative. Adv. Mater. 2009, 21, 2050–2053. [Google Scholar] [CrossRef]
- Saccone, M.; Pfletscher, M.; Dautzenberg, E.; Dong, R.Y.; Michal, C.A.; Giese, M. Hydrogen-bonded liquid crystals with broad-range blue phases. J. Mater. Chem. C 2019, 7, 3150–3153. [Google Scholar] [CrossRef]
- Monte, M.J.S.; Almeida, A.R.R.P.; Ribeiro da Silva, M.A.V. Thermodynamic study of the sublimation of eight 4-n-alkylbenzoic acids. J. Chem. Thermodyn. 2004, 36, 385–392. [Google Scholar] [CrossRef]
- Gray, G.W.; McDonnell, D.G. Liquid Crystal Compounds Incorporating the Trans-1,4-Substituted Cyclohexane Ring System. Mol. Cryst. Liq. Cryst. 1979, 53, 147–166. [Google Scholar] [CrossRef]
- Nayani, K.; Rai, P.; Bao, N.; Yu, H.; Mavrikakis, M.; Twieg, R.J.; Abbott, N.L. Liquid Crystals with Interfacial Ordering that Enhances Responsiveness to Chemical Targets. Adv. Mater. 2018, 30, 1706707. [Google Scholar] [CrossRef]
- Cao, L.; Sun, G.; Zhang, C.; Liu, W.; Li, J.; Wang, L. An Intelligent Film Based on Cassia Gum Containing Bromothymol Blue-Anchored Cellulose Fibers for Real-Time Detection of Meat Freshness. J. Agric. Food Chem. 2019, 67, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Y.; Yuan, J.; Li, Z.; Zhao, R.; Han, T.; Han, T. To direct the self-assembly of AIEgens by three-gear switch: Morphology study, amine sensing and assessment of meat spoilage. Sens. Actuators B Chem. 2018, 258, 373–380. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, H.; Abbott, N.L.; Zavala, V.M. Machine Learning Algorithms for Liquid Crystal-Based Sensors. ACS Sens. 2018, 3, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Abbott, N.; Zavala, V.M. Convolutional Network Analysis of Optical Micrographs for Liquid Crystal Sensors. J. Phys. Chem. C 2020, 124, 15152–15161. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, X.; Zhang, Y.; Che, Y.; Zhao, J. Detection of Amines with Fluorescent Nanotubes: Applications in the Assessment of Meat Spoilage. ACS Sensors 2016, 1, 22–25. [Google Scholar] [CrossRef]
- Bedolla Pantoja, M.A.; Abbott, N.L. Surface-Controlled Orientational Transitions in Elastically Strained Films of Liquid Crystal That Are Triggered by Vapors of Toluene. ACS Appl. Mater. Interfaces 2016, 8, 13114–13122. [Google Scholar] [CrossRef]
- Miller, D.S.; Carlton, R.J.; Mushenheim, P.C.; Abbott, N.L. Introduction to optical methods for characterizing liquid crystals at interfaces. Langmuir 2013, 29, 3154–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, J.T.; Abbott, N.L. Dynamics of the chemo-optical response of supported films of nematic liquid crystals. Sensors Actuators B Chem. 2013, 183, 71–80. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Perdew, J.P.; Burken, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Szilvási, T.; Bao, N.; Yu, H.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. The role of anions in adsorbate-induced anchoring transitions of liquid crystals on surfaces with discrete cation binding sites. Soft Matter 2018, 14, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Szilvási, T.; Roling, L.T.; Yu, H.; Rai, P.; Choi, S.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Design of Chemoresponsive Liquid Crystals through Integration of Computational Chemistry and Experimental Studies. Chem. Mater. 2017, 29, 3563–3571. [Google Scholar] [CrossRef]
- Wang, K.; Rai, P.; Fernando, A.; Szilvási, T.; Yu, H.; Abbott, N.L.; Mavrikakis, M.; Twieg, R.J. Synthesis and properties of fluorine tail-terminated cyanobiphenyls and terphenyls for chemoresponsive liquid crystals. Liq. Cryst. 2019, 1–14. [Google Scholar] [CrossRef]
- Wang, K.; Jirka, M.; Rai, P.; Twieg, R.J.; Szilvási, T.; Yu, H.; Abbott, N.L.; Mavrikakis, M. Synthesis and properties of hydroxy tail-terminated cyanobiphenyl liquid crystals. Liq. Cryst. 2019, 46, 397–407. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Website. Available online: https://www.tcichemicals.com/AU/en/p/P0944#docomentsSectionPDP (accessed on 22 October 2020).
- Kirsch, P.; Bremer, M. Nematic liquid crystals for active matrix displays: Molecular design and synthesis. Angew. Chemie Int. Ed. 2000, 39, 4216–4235. [Google Scholar] [CrossRef]
- Rajanandkumar, R.; Prabu, N.P.S.; Mohan, M.L.N.M. Characterization of Hydrogen Bonded Liquid Crystals Formed by Suberic Acid and Alkyl Benzoic Acids. Mol. Cryst. Liq. Cryst. 2013, 587, 60–79. [Google Scholar] [CrossRef]
- Otero, V.; Sanches, D.; Montagner, C.; Vilarigues, M.; Carlyle, L.; Lopes, J.A.; Melo, M.J. Characterisation of metal carboxylates by Raman and infrared spectroscopy in works of art. J. Raman Spectrosc. 2014, 45, 1197–1206. [Google Scholar] [CrossRef]
- Karabacak, M.; Cinar, Z.; Kurt, M.; Sudha, S.; Sundaraganesan, N. FT-IR, FT-Raman, NMR and UV–vis spectra, vibrational assignments and DFT calculations of 4-butyl benzoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 85, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, V.; Mathammal, R. Density functional and experimental studies on the FT-IR and FT-Raman spectra and structure of benzoic acid and 3,5-dichloro salicylic acid. J. Raman Spectrosc. 2009, 40, 264–271. [Google Scholar] [CrossRef]
- Long, M.; Zhang, T.; Chai, Y.; Ng, C.F.; Mak, T.C.W.; Xu, J.; Yan, K. Nonstoichiometric acid-base reaction as reliable synthetic route to highly stable CH3NH3PbI3 perovskite film. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Bruckenstein, S.; Untereker, D.F. Acid-Base Reactions between Amines and Carboxylic Acids in Hexane. J. Am. Chem. Soc. 1969, 91, 5741–5745. [Google Scholar] [CrossRef]
- Pan, R.P.; Tsai, T.R.; Chen, C.Y.; Pan, C.L. Optical constants of two typical liquid crystals 5CB and PCH5 in the THz frequency range. J. Biol. Phys. 2003, 29, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Apelblat, A.; Manzurola, E.; Abo Balal, N. The solubilities of benzene polycarboxylic acids in water. J. Chem. Thermodyn. 2006, 38, 565–571. [Google Scholar] [CrossRef]
- Chen, J.; Brooks, C.L.; Scheraga, H.A. Revisiting the carboxylic acid dimers in aqueous solution: Interplay of hydrogen bonding, hydrophobic interactions and entropy. J. Phys. Chem. B 2008, 112, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Nishi, N. Hydrophobic Hydration and Hydrophobic Interaction of Carboxylic Acids in Aqueous Solution: Mass Spectrometric Analysis of Liquid Fragments Isolated as Clusters. J. Am. Chem. Soc. 1990, 112, 549–558. [Google Scholar] [CrossRef]
- Ng, J.B.; Shurvell, H.F. Application of factor analysis and band contour resolution techniques to the Raman spectra of acetic acid in aqueous solution. J. Phys. Chem. 1987, 91, 496–500. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, Y.K.; Wang, X.; Tsuei, M.; Abbott, N.L. Structural and Optical Response of Polymer-Stabilized Blue Phase Liquid Crystal Films to Volatile Organic Compounds. ACS Appl. Mater. Interfaces 2020, 12, 42099–42108. [Google Scholar] [CrossRef]







| Frequency | C4BA | C5CA | C4BA + C5CA | TEA |
|---|---|---|---|---|
| ν(C=O) | 1680 (1680) | 1693 (1693) | 1687 (1689) | |
| ν(C-C)ring | 1610, 1576 | 1608, 1573 | ||
| β(O-H) | 1425 | 1425 | 1421 | |
| νas(COO−) | 1545 (1541) | 1558 (1562) | 1547 | |
| β(C-H) | 1465, 1454 | 1469, 1448 | 1463, 1450 | 1469, 1448 |
| ν(C-N) | 1381 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wang, K.; Szilvási, T.; Nayani, K.; Bao, N.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Design of Chemoresponsive Soft Matter Using Hydrogen-Bonded Liquid Crystals. Materials 2021, 14, 1055. https://doi.org/10.3390/ma14051055
Yu H, Wang K, Szilvási T, Nayani K, Bao N, Twieg RJ, Mavrikakis M, Abbott NL. Design of Chemoresponsive Soft Matter Using Hydrogen-Bonded Liquid Crystals. Materials. 2021; 14(5):1055. https://doi.org/10.3390/ma14051055
Chicago/Turabian StyleYu, Huaizhe, Kunlun Wang, Tibor Szilvási, Karthik Nayani, Nanqi Bao, Robert J. Twieg, Manos Mavrikakis, and Nicholas L. Abbott. 2021. "Design of Chemoresponsive Soft Matter Using Hydrogen-Bonded Liquid Crystals" Materials 14, no. 5: 1055. https://doi.org/10.3390/ma14051055
APA StyleYu, H., Wang, K., Szilvási, T., Nayani, K., Bao, N., Twieg, R. J., Mavrikakis, M., & Abbott, N. L. (2021). Design of Chemoresponsive Soft Matter Using Hydrogen-Bonded Liquid Crystals. Materials, 14(5), 1055. https://doi.org/10.3390/ma14051055