Fe(III) Ions-Assisted Aniline Polymerization Strategy to Nitrogen-Doped Carbon-Supported Bimetallic CoFeP Nanospheres as Efficient Bifunctional Electrocatalysts toward Overall Water Splitting
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of CoFeOx-PANI
2.3. Synthesis of CoFeP-NC
2.4. Characterization
2.5. Electrochemical Measurements
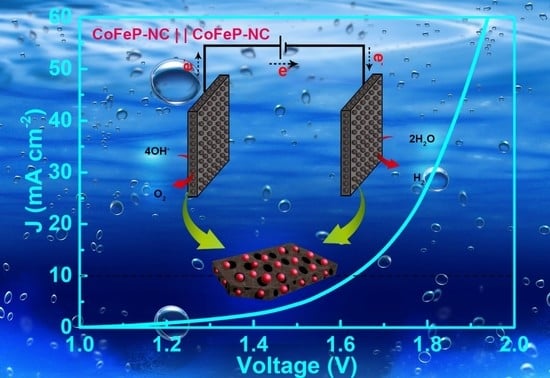
3. Results and Discussions
3.1. Characterization
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Zheng, B.; Wang, B.; Wang, H.; Sun, B.; He, J.; Zhang, W.; Chen, Y. Hierarchical MoSe2-CoSe2 nanotubes anchored on graphene nanosheets: A highly efficient and stable electrocatalyst for hydrogen evolution in alkaline medium. Electrochim. Acta 2019, 299, 197–205. [Google Scholar] [CrossRef]
- Yang, H.; Tang, Z.; Wang, K.; Wu, W.; Chen, Y.; Ding, Z.; Liu, Z.; Chen, S. Co@Pd core-shell nanoparticles embedded in nitrogen-doped porous carbon as dual functional electrocatalysts for both oxygen reduction and hydrogen evolution reactions. J. Colloid Interface Sci. 2018, 528, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Ma, F.X.; Xu, C.Y.; Lyu, F.; Song, B.; Sun, S.C.; Li, Y.Y.; Lu, J.; Zhen, L. Construction of FeP Hollow Nanoparticles Densely Encapsulated in Carbon Nanosheet Frameworks for Efficient and Durable Electrocatalytic Hydrogen Production. Adv. Sci. 2019, 6, 1801490. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Wang, J.; Teng, X.; Meyer, T.J.; Chen, Z. CoP Nanoframes as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. ACS Catal. 2019, 10, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Kundu, A.; Robby, A.I.; Shit, A.; Jo, H.J.; Park, S.Y. Construction of FeCo2O4@N-Doped Carbon Dots Nanoflowers as Binder Free Electrode for Reduction and Oxidation of Water. Materials 2020, 13, 3119. [Google Scholar] [CrossRef]
- Li, R.-Q.; Hu, P.; Miao, M.; Li, Y.; Jiang, X.-F.; Wu, Q.; Meng, Z.; Hu, Z.; Bando, Y.; Wang, X.-B. CoO-modified Co4N as a heterostructured electrocatalyst for highly efficient overall water splitting in neutral media. J. Mater. Chem. A 2018, 6, 24767–24772. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chen, L.; Xu, H.; Xiong, Y. 2D Polymers as Emerging Materials for Photocatalytic Overall Water Splitting. Adv. Mater. 2018, 30, 1801955. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Zhong, Y.; Bu, Y.; Chen, X.; Zhong, Q.; Liu, M.; Shao, Z. A Perovskite Nanorod as Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602122. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, Z.; Fu, H.; Zhang, X.; Tian, G.; Fu, H. NiSe-Ni0.85 Se Heterostructure Nanoflake Arrays on Carbon Paper as Efficient Electrocatalysts for Overall Water Splitting. Small 2018, 14, 1800763. [Google Scholar] [CrossRef]
- Ding, J.; Wang, P.; Ji, S.; Wang, H.; Brett, D.J.L.; Wang, R. Mesoporous nickel selenide N-doped carbon as a robust electrocatalyst for overall water splitting. Electrochim. Acta 2019, 300, 93–101. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, Z.; Chen, X.; Li, Y. A KCl-assisted pyrolysis strategy to fabricate nitrogen-doped carbon nanotube hollow polyhedra for efficient bifunctional oxygen electrocatalysts. J. Mater. Chem. A 2019, 7, 20310–20316. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, C.; Hu, E.; Xia, B.; Ning, J.; Zheng, C.; Zhong, Y.; Zhang, Z.; Hu, Y. Construction of hierarchical FeP/Ni2P hollow nanospindles for efficient oxygen evolution. J. Mater. Chem. A 2018, 6, 14103–14111. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, W.J.; Wei, Z.; Tang, T.; Ma, J.; Hu, J.S.; Wan, L.J. Se-Doping Activates FeOOH for Cost-Effective and Efficient Electrochemical Water Oxidation. J. Am. Chem. Soc. 2019, 141, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel Co3O4 Nanoparticles/Nitrogen-Doped Carbon Composites with Extraordinary Catalytic Activity for Oxygen Evolution Reaction (OER). Nanomicro Lett 2018, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Li, T.-T.; Qian, J.; Hu, Y.; Guo, F.; Zheng, Y.-Q. Self-supported hierarchical CuOx@Co3O4 heterostructures as efficient bifunctional electrocatalysts for water splitting. J. Mater. Chem. A 2018, 6, 14431–14439. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Z.N.; Li, L.; Yin, P.; Liu, Z.; Zhang, H. Ultrathin Ni(0)-Embedded Ni(OH)2 Heterostructured Nanosheets with Enhanced Electrochemical Overall Water Splitting. Adv. Mater. 2020, 32, 1906915. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, K.; Shi, K.; Fu, Z.; Mai, Z.; Wang, X.; Tang, Z.; Tian, Y. Interfacial electron transfer of heterostructured MIL-88A/Ni(OH)2 enhances the oxygen evolution reaction in alkaline solutions. J. Mater. Chem. A 2020, 8, 3311–3321. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Park, S.-K.; Kim, J.K.; Chan Kang, Y. Metal–organic framework-derived CoSe2/(NiCo)Se2 box-in-box hollow nanocubes with enhanced electrochemical properties for sodium-ion storage and hydrogen evolution. J. Mater. Chem. A 2017, 5, 18823–18830. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Yu, B.; Sun, B.; Yang, D.; Zhang, X.; Zhang, Q.; Zhang, W.; Gu, L.; Chen, Y. CoSe2 nanoparticles embedded MOF-derived Co-N-C nanoflake arrays as efficient and stable electrocatalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 258, 117996. [Google Scholar] [CrossRef]
- Xia, Z.; Sun, H.; He, X.; Sun, Z.; Lu, C.; Li, J.; Peng, Y.; Dou, S.; Sun, J.; Liu, Z. In situ construction of CoSe2@vertical-oriented graphene arrays as self-supporting electrodes for sodium-ion capacitors and electrocatalytic oxygen evolution. Nano Energy 2019, 60, 385–393. [Google Scholar] [CrossRef]
- Liang, H.; Yang, C.; Ji, S.; Jiang, N.; An, X.; Yang, X.; Wang, H.; Wang, R. Cobalt-nickel phosphides@carbon spheres as highly efficient and stable electrocatalyst for hydrogen evolution reaction. Catal. Commun. 2019, 124, 1–5. [Google Scholar] [CrossRef]
- Du, X.; Su, H.; Zhang, X. Metal-organic framework-derived M (M = Fe, Ni, Zn and Mo) doped Co9S8 nanoarrays as efficient electrocatalyst for water splitting: The combination of theoretical calculation and experiment. J. Catal. 2020, 383, 103–116. [Google Scholar] [CrossRef]
- He, L.; Zhou, D.; Lin, Y.; Ge, R.; Hou, X.; Sun, X.; Zheng, C. Ultrarapid in Situ Synthesis of Cu2S Nanosheet Arrays on Copper Foam with Room-Temperature-Active Iodine Plasma for Efficient and Cost-Effective Oxygen Evolution. ACS Catal. 2018, 8, 3859–3864. [Google Scholar] [CrossRef]
- Li, L.; Song, L.; Guo, H.; Xia, W.; Jiang, C.; Gao, B.; Wu, C.; Wang, T.; He, J. N-Doped porous carbon nanosheets decorated with graphitized carbon layer encapsulated Co9S8 nanoparticles: An efficient bifunctional electrocatalyst for the OER and ORR. Nanoscale 2019, 11, 901–907. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Sun, Y.; Qi, K.; Jin, Z.; Wei, S.; Li, W.; Zhang, L.; Zheng, W. Nanoporous Sulfur-Doped Copper Oxide (Cu2OxS1-x) for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 745–752. [Google Scholar] [CrossRef]
- Wu, A.; Xie, Y.; Ma, H.; Tian, C.; Gu, Y.; Yan, H.; Zhang, X.; Yang, G.; Fu, H. Integrating the active OER and HER components as the heterostructures for the efficient overall water splitting. Nano Energy 2018, 44, 353–363. [Google Scholar] [CrossRef]
- Ma, J.; Wang, M.; Lei, G.; Zhang, G.; Zhang, F.; Peng, W.; Fan, X.; Li, Y. Polyaniline Derived N-Doped Carbon-Coated Cobalt Phosphide Nanoparticles Deposited on N-Doped Graphene as an Efficient Electrocatalyst for Hydrogen Evolution Reaction. Small 2018, 14, 1702895. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Qu, H.; Liu, Y.; Han, Y.; Wang, L.; Dong, B. Bimetallic CoFeP hollow microspheres as highly efficient bifunctional electrocatalysts for overall water splitting in alkaline media. Appl. Surf. Sci. 2019, 465, 816–823. [Google Scholar] [CrossRef]
- Yu, L.; Xiao, Y.; Luan, C.; Yang, J.; Qiao, H.; Wang, Y.; Zhang, X.; Dai, X.; Yang, Y.; Zhao, H. Cobalt/Molybdenum Phosphide and Oxide Heterostructures Encapsulated in N-Doped Carbon Nanocomposite for Overall Water Splitting in Alkaline Media. ACS Appl. Mater. Interfaces 2019, 11, 6890–6899. [Google Scholar] [CrossRef]
- Sun, Q.; Tong, Y.; Chen, P.; Chen, L.; Xi, F.; Liu, J.; Dong, X. Dual anions engineering on nickel cobalt-based catalyst for optimal hydrogen evolution electrocatalysis. J. Colloid Interface Sci. 2021, 589, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lu, J.; Zhou, K.; Yang, L.; Ke, Y.; Tang, Z.; Chen, S. CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy 2016, 28, 143–150. [Google Scholar] [CrossRef]
- Hao, Y.; Xu, Y.; Liu, W.; Sun, X. Co/CoP embedded in a hairy nitrogen-doped carbon polyhedron as an advanced tri-functional electrocatalyst. Mater. Horiz. 2018, 5, 108–115. [Google Scholar] [CrossRef]
- Zhai, M.; Wang, F.; Du, H. Transition-Metal Phosphide-Carbon Nanosheet Composites Derived from Two-Dimensional Metal-Organic Frameworks for Highly Efficient Electrocatalytic Water-Splitting. ACS Appl. Mater. Interfaces 2017, 9, 40171–40179. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Liang, X.; Zhuang, Z. Multishelled FeCo@FeCoP@C Hollow Spheres as Highly Efficient Hydrogen Evolution Catalysts. ACS Appl. Mater. Interfaces 2018, 11, 1267–1273. [Google Scholar] [CrossRef]
- Pi, C.; Huang, C.; Yang, Y.; Song, H.; Zhang, X.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K.; Huo, K. In situ formation of N-doped carbon-coated porous MoP nanowires: A highly efficient electrocatalyst for hydrogen evolution reaction in a wide pH range. Appl. Catal. B Environ. 2020, 263, 118358. [Google Scholar] [CrossRef]
- Niu, W.; Li, L.; Liu, X.; Wang, N.; Liu, J.; Zhou, W.; Tang, Z.; Chen, S. Mesoporous N-doped carbons prepared with thermally removable nanoparticle templates: An efficient electrocatalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 5555–5562. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, L.; Bukhvalov, D.; Chen, Z.; Zou, Z.; Shang, L.; Yang, X.; Yan, D.; Han, F.; Zhang, T. Hierarchical ultrathin carbon encapsulating transition metal doped MoP electrocatalysts for efficient and pH-universal hydrogen evolution reaction. Nano Energy 2020, 70, 104445. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, E.; Yan, Q.; Hu, Z.; Xiao, X.; Chen, D. The Effect of Crystal Face of Fe2O3 on the Electrochemical Performance for Lithium-ion Batteries. Sci. Rep. 2016, 6, 29381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Dong, C.L.; Zou, Y.; Su, D.; Huang, Y.C.; Tao, L.; Dou, S.; Shen, S.; Wang, S. In situ evolution of highly dispersed amorphous CoOx clusters for oxygen evolution reaction. Nanoscale 2017, 9, 11969–11975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Wang, Q.; Li, Y.; Zhang, C. Amorphous cobalt-cerium binary metal oxides as high performance electrocatalyst for oxygen evolution reaction. J. Catal. 2020, 384, 14–21. [Google Scholar] [CrossRef]
- Yue, S.; Wang, S.; Jiao, Q.; Feng, X.; Zhan, K.; Dai, Y.; Feng, C.; Li, H.; Feng, T.; Zhao, Y. Preparation of Yolk-Shell-Structured Cox Fe1-x P with Enhanced OER Performance. ChemSusChem 2019, 12, 4461–4470. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, M.; Jiang, Y.-Z.; Wang, M.; Li, Z.-Y.; Zhou, Z. Metal-organic-framework-derived porous 3D heterogeneous NiFex/NiFe2O4@NC nanoflowers as highly stable and efficient electrocatalysts for the oxygen-evolution reaction. J. Mater. Chem. A 2019, 7, 21338–21348. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, D.; Zhou, W.; Xu, W.; Liang, X.; He, G.; Wu, Z.; Li, L. Nitrogen-Doped Hollow Carbon Polyhedrons with Carbon Nanotubes Surface Layers as Effective Sulfur Hosts for High-Rate, Long-Lifespan Lithium–Sulfur Batteries. ChemElectroChem 2020, 7, 4990–4998. [Google Scholar] [CrossRef]
- Cao, L.M.; Hu, Y.W.; Tang, S.F.; Iljin, A.; Wang, J.W.; Zhang, Z.M.; Lu, T.B. Fe-CoP Electrocatalyst Derived from a Bimetallic Prussian Blue Analogue for Large-Current-Density Oxygen Evolution and Overall Water Splitting. Adv. Sci. 2018, 5, 1800949. [Google Scholar] [CrossRef]
- Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, H.Y. Integration of Cobalt Metal–Organic Frameworks into an Interpenetrated Prussian Blue Analogue to Derive Dual Metal–Organic Framework-Assisted Cobalt Iron Derivatives for Enhancing Electrochemical Total Water Splitting. J. Phys. Chem. C 2020, 124, 14465–14476. [Google Scholar] [CrossRef]
- Niu, Z.; Qiu, C.; Jiang, J.; Ai, L. Hierarchical CoP–FeP Branched Heterostructures for Highly Efficient Electrocatalytic Water Splitting. ACS Sustain. Chem. Eng. 2018, 7, 2335–2342. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Li, A.; Zheng, X.; Peng, L.; Huang, J.; Deng, Z.; Chen, H.; Wei, Z. Rational construction of macroporous CoFeP triangular plate arrays from bimetal–organic frameworks as high-performance overall water-splitting catalysts. J. Mater. Chem. A 2019, 7, 17529–17535. [Google Scholar] [CrossRef]
- Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, H.Y. Zeolitic imidazolate framework derived Co3S4 hybridized MoS2–Ni3S2 heterointerface for electrochemical overall water splitting reactions. Electrochim. Acta 2020, 334, 135537. [Google Scholar] [CrossRef]
- Ryu, J.; Jung, N.; Jang, J.H.; Kim, H.-J.; Yoo, S.J. In Situ Transformation of Hydrogen-Evolving CoP Nanoparticles: Toward Efficient Oxygen Evolution Catalysts Bearing Dispersed Morphologies with Co-oxo/hydroxo Molecular Units. ACS Catal. 2015, 5, 4066–4074. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wei, F.; Lv, H.; Zhao, D.; Wang, N.; Li, L.; Li, N.; Wang, X. Fe(III) Ions-Assisted Aniline Polymerization Strategy to Nitrogen-Doped Carbon-Supported Bimetallic CoFeP Nanospheres as Efficient Bifunctional Electrocatalysts toward Overall Water Splitting. Materials 2021, 14, 1473. https://doi.org/10.3390/ma14061473
Zhao C, Wei F, Lv H, Zhao D, Wang N, Li L, Li N, Wang X. Fe(III) Ions-Assisted Aniline Polymerization Strategy to Nitrogen-Doped Carbon-Supported Bimetallic CoFeP Nanospheres as Efficient Bifunctional Electrocatalysts toward Overall Water Splitting. Materials. 2021; 14(6):1473. https://doi.org/10.3390/ma14061473
Chicago/Turabian StyleZhao, Changhao, Fen Wei, Haolin Lv, Dengke Zhao, Nan Wang, Ligui Li, Nanwen Li, and Xiufang Wang. 2021. "Fe(III) Ions-Assisted Aniline Polymerization Strategy to Nitrogen-Doped Carbon-Supported Bimetallic CoFeP Nanospheres as Efficient Bifunctional Electrocatalysts toward Overall Water Splitting" Materials 14, no. 6: 1473. https://doi.org/10.3390/ma14061473
APA StyleZhao, C., Wei, F., Lv, H., Zhao, D., Wang, N., Li, L., Li, N., & Wang, X. (2021). Fe(III) Ions-Assisted Aniline Polymerization Strategy to Nitrogen-Doped Carbon-Supported Bimetallic CoFeP Nanospheres as Efficient Bifunctional Electrocatalysts toward Overall Water Splitting. Materials, 14(6), 1473. https://doi.org/10.3390/ma14061473