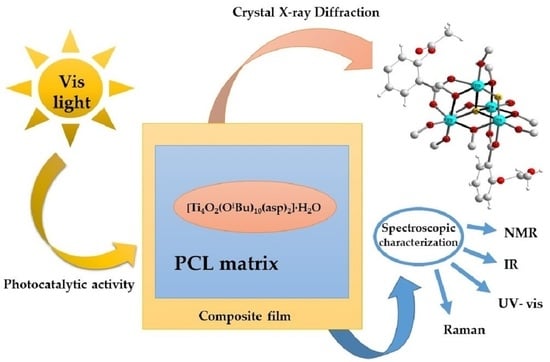
Titanium(IV) Oxo-Complex with Acetylsalicylic Acid Ligand and Its Polymer Composites: Synthesis, Structure, Spectroscopic Characterization, and Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Tetranuclear Ti(IV)-Oxo Complex (1) Stabilized by Acetylsalicylic Ligands and the PCL + (1) Composite
2.3. Analytical Procedures
2.3.1. Single Crystal X-ray Diffraction Measurement
2.3.2. Spectroscopic Characterization
2.3.3. The Photocatalytic Activity Evaluation of (PCL + TOCs) Composites
3. Results
3.1. Structure of [Ti4O2(asp)2(BuiO)10]·H2O (asp = O2C-o-PhO2CCH3) (1)
3.2. NMR Spectroscopy
3.3. Analysis of Vibrational Spectra
3.4. UV–Vis Diffuse Reflectance Spectra (UV–Vis DRS) of the Oxo-Complex and HOMO–LUMO Gap Determination
3.5. The Films of Poly(Caprolactone) and (1) Composite
3.6. Thermal Analysis of PCL + (1) Composites
3.7. Estimation of Photocatalytic Activity of the Oxo-Complexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Environmental Application of Photocatalysis. Mater. Sci. Forum 2012, 734, 273–294. [Google Scholar] [CrossRef]
- Coppens, P.; Chen, Y.; Trzop, E. Crystallography and Properties of Polyoxotitanate Nanoclusters. Chem. Rev. 2014, 114, 9645–9661. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhu, Y.-F.; Chen, Z.-H.; Liu, F.-H.; Zhao, L.; Su, Z.-M. Synthesis, structure, and photocatalytic hydrogen of three environmentally friendly titanium oxo-clusters. Inorg. Chem. Commun. 2014, 40, 22–25. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Luo, W.; Wang, Y.-H.; Pu, Y.-Y.; Zhang, X.; You, L.-S.; Zhu, Q.-Y.; Dai, J. Titanium–oxo–Clusters with Dicarboxylates: Single-Crystal Structure and Photochromic Effect. Inorg. Chem. 2012, 51, 8982–8988. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Lu, X.-W.; Qi, M.; Su, H.-C.; Zhao, X.-W.; Zhu, Q.-Y.; Dai, J. Titanium–Oxo Cluster with 9-Anthracenecarboxylate Antennae: A Fluorescent and Photocurrent Transfer Material. Inorg. Chem. 2014, 53, 7233–7240. [Google Scholar] [CrossRef]
- Yin, J.-X.; Huo, P.; Wang, S.; Wu, J.; Zhu, Q.-Y.; Dai, J. A tetrathiafulvalene-grafted titanium-oxo-cluster material: Self-catalyzed crystal exfoliation and photocurrent response properties. J. Mater. Chem. C 2014, 3, 409–415. [Google Scholar] [CrossRef]
- Kubiak, B.; Radtke, A.; Topolski, A.; Wrzeszcz, G.; Golińska, P.; Kaszkowiak, E.; Sobota, M.; Włodarczyk, J.; Stojko, M.; Piszczek, P. The Composites of PCL and Tetranuclear Titanium(IV)-oxo Complexes as Materials Exhibiting the Photocatalytic and the Antimicrobial Activity. Int. J. Mol. Sci. 2021, 22, 7021. [Google Scholar] [CrossRef]
- Piszczek, P.; Kubiak, B.; Golińska, P.; Radtke, A. Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test. Int. J. Mol. Sci. 2020, 21, 9663. [Google Scholar] [CrossRef]
- Assi, H.; Mouchaham, G.; Steunou, N.; Devic, T.; Serre, C. Titanium coordination compounds: From discrete metal complexes to metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 3431–3452. [Google Scholar] [CrossRef]
- Benedict, J.B.; Freindorf, R.; Trzop, E.; Cogswell, J.; Coppens, P. Large Polyoxotitanate Clusters: Well-Defined Models for Pure-Phase TiO2 Structures and Surfaces. J. Am. Chem. Soc. 2010, 132, 13669–13671. [Google Scholar] [CrossRef] [PubMed]
- Rozes, L.; Sanchez, C. Titanium oxo-clusters: Precursors for a Lego-like construction of nanostructured hybrid materials. Chem. Soc. Rev. 2011, 40, 1006–1030. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.S.; Luo, H.; Li, N.; Matthews, P.D. Novel properties and potential applications of functional ligand-modified polyoxotitanate cages–Chemical Communications (RSC Publishing). Chem. Commun. 2016, 52, 11180–11190. [Google Scholar] [CrossRef] [Green Version]
- Janek, M.; Radtke, A.; Muzioł, T.M.; Jerzykiewicz, M.; Piszczek, P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials 2018, 11, 1661. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.-H.; Zhang, L.; Zhang, J. Synthetic strategies, diverse structures and tuneable properties of polyoxo-titanium clusters. Chem. Soc. Rev. 2018, 47, 404–421. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, L.; Zhang, J. Acid-Controlled Synthesis of Carboxylate-Stabilized Ti44 -Oxo Clusters: Scaling up Preparation, Exchangeable Protecting Ligands, and Photophysical Properties. Chem. A Eur. J. 2019, 25, 10450–10455. [Google Scholar] [CrossRef]
- Schubert, U. Chemical modification of titanium alkoxides for sol-gel processing. J. Mater. Chem. 2005, 15, 3701–3715. [Google Scholar] [CrossRef]
- Radtke, A.; Piszczek, P.; Muzioł, T.; Wojtczak, A. The Structural Conversion of Multinuclear Titanium(IV) μ-Oxo-complexes. Inorg. Chem. 2014, 53, 10803–10810. [Google Scholar] [CrossRef]
- Janek, M.; Muzioł, T.; Piszczek, P. The structure and photocatalytic activity of the tetranuclear titanium(IV) oxo-complex with 4-aminobenzoate ligands. Polyhedron 2017, 141, 110–117. [Google Scholar] [CrossRef]
- Janek, M.; Muzioł, T.M.; Piszczek, P. Trinuclear Oxo-Titanium Clusters: Synthesis, Structure, and Photocatalytic Activity. Materials 2019, 12, 3195. [Google Scholar] [CrossRef] [Green Version]
- Schubert, U. Titanium-Oxo Clusters with Bi- and Tridentate Organic Ligands: Gradual Evolution of the Structures from Small to Big. Chem. A Eur. J. 2021, 27, 11239–11256. [Google Scholar] [CrossRef] [PubMed]
- Torsney, E.; Mayr, U.; Zou, Y.; Thompson, W.D.; Hu, Y.; Xu, Q. Thrombosis and Neointima Formation in Vein Grafts Are Inhibited by Locally Applied Aspirin Through Endothelial Protection. Circ. Res. 2004, 94, 1466–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akşit, E.; Kurt, T.; Büyük, B.; Çokkalender, Ö. Drug-eluting Vein Graft with Acetylsalicylic Acid-Ticagrelor-Unfractionated Heparin Complex Inhibits Early Graft Thrombosis. Balk. Med. J. 2020, 37, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Schrör, K. Acetylsalicylic Acid; John Wiley & Sons: Weinheim, Germany, 2016; ISBN 9783527685028. [Google Scholar]
- Dave, R.N.; Joshi, H.M.; Venugopalan, V.P. Novel Biocatalytic Polymer-Based Antimicrobial Coatings as Potential Ureteral Biomaterial: Preparation and In Vitro Performance Evaluation. Antimicrob. Agents Chemother. 2011, 55, 845–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Low, J.; Kao, P.H.-N.; Tambyah, P.A.; Koh, G.L.E.; Ling, H.; Kline, K.A.; Cheow, W.S.; Leong, S.S.J. Development of a polymer-based antimicrobial coating for efficacious urinary catheter protection. Biotechnol. Notes 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Meth-Cohn, O.; Thorpe, D.; Twitchett, H.J. Insertion reactions of titanium alkoxides with isocyanates and carbodiimides. J. Chem. Soc. C Org. 1970, 132–135. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Krug, M.; Weiss, M.S.; Heinemann, U.; Mueller, U. XDSAPP: A graphical user interface for the convenient processing of diffraction data using XDS. J. Appl. Crystallogr. 2012, 45, 568–572. [Google Scholar] [CrossRef]
- E. CrysAlis Red and CrysAlis CCD; Oxford Diffraction Ltd.: Abingdon, UK, 2000.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K.; Berndt, M. Diamond, Release 2.1e; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- ISO 10678:2010(E) Standards; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Pho-tocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. ISO: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/46019.html (accessed on 1 May 2022).
- Ehlert, M.; Radtke, A.; Topolski, A.; Śmigiel, J.; Piszczek, P. The Photocatalytic Activity of Titania Coatings Produced by Electrochemical and Chemical Oxidation of Ti6Al4V Substrate, Estimated According to ISO 10678:2010. Materials 2020, 13, 2649. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.S.K.; Kumar, T.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and Bioactive Surface Modifications of Titanium Implants by PCL/TiO2 Nanocomposite Coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Xiong, X.; Chen, W. Cryogenic Raman Spectroscopic Studies on Common Ore-forming Fluid Systems. Minerals 2019, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Muthuselvi, C.; Dhavachitra, M.; Pandiarajan, S. Growth and Characterization of Aspirin Crystal in the Phosphoric Acid Medium. J. Chem. Pharm. Res. 2017, 8, 804–814. [Google Scholar]
- Wendlandt, W.W.; Hecht, H.G. Reflectance Spectroscopy; Interscience Publishers: New York, NY, USA, 1966; pp. 214–281. [Google Scholar]
- Piszczek, P.; Radtke, A.; Muzioł, T.; Richert, M.; Chojnacki, J. The conversion of multinuclear μ-oxo titanium(iv) species in the reaction of Ti(OiBu)4 with branched organic acids; results of structural and spectroscopic studies. Dalton Trans. 2012, 41, 8261–8269. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, C.; Chaumont, A.; Kyritsakas, N.; Mobian, P.; Henry, M. Titanium oxo-clusters derivatized from the Ti10O12(cat)8(py)8 complex: Structural investigation and spectroscopic studies of light absorption. Dalton Trans. 2016, 45, 8760–8769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-X.; Gao, M.-Y.; Fang, W.-H.; Zhang, L.; Zhang, J. Bandgap Engineering of Titanium-Oxo Clusters: Labile Surface Sites Used for Ligand Substitution and Metal Incorporation. Angew. Chem. Int. Ed. 2016, 55, 5160–5165. [Google Scholar] [CrossRef]
- Hong, Z.-F.; Xu, S.-H.; Yan, Z.-H.; Lu, D.-F.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. A Large Titanium Oxo Cluster Featuring a Well-Defined Structural Unit of Rutile. Cryst. Growth Des. 2018, 18, 4864–4868. [Google Scholar] [CrossRef]
- Angarano, V.; Smet, C.; Akkermans, S.; Watt, C.; Chieffi, A.; Van Impe, J.F.M. Visible Light as an Antimicrobial Strategy for Inactivation of Pseudomonas fluorescens and Staphylococcus epidermidis Biofilms. Antibiotics 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubart, R.; Lipovski, A.; Nitzan, Y.; Friedmann, H. A possible mechanism for the bactericidal effect of visible light. Laser Ther. 2011, 20, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanam, N.; Chintakrinda, K.; Fang, W.-H.; Kang, Y.; Zhang, L.; Zhang, J. Azole Functionalized Polyoxo-Titanium Clusters with Sunlight-Driven Dye Degradation Applications: Synthesis, Structure, and Photocatalytic Studies. Inorg. Chem. 2016, 55, 10294–10301. [Google Scholar] [CrossRef]
- Wang, J.-F.; Fang, W.-H.; Li, D.-S.; Zhang, L.; Zhang, J. Cocrystal of {Ti4} and {Ti6} Clusters with Enhanced Photochemical Properties. Inorg. Chem. 2017, 56, 2367–2370. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Gohari, G.; Mohammadi, A.; Akbari, A.; Panahirad, S.; Dadpour, M.R.; Fotopoulos, V.; Kimura, S. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 2020, 10, 912. [Google Scholar] [CrossRef]
- Bagheri, S.; Shameli, K.; Abd Hamid, S.B. Synthesis and Characterization of Anatase Titanium Dioxide Nanoparticles Using Egg White Solution via Sol-Gel Method. J. Chem. 2013, 2013, 848205. [Google Scholar] [CrossRef]
- Erdal, N.B.; Lando, G.A.; Yadav, A.; Srivastava, R.K.; Hakkarainen, M. Hydrolytic Degradation of Porous Crosslinked Poly(ε-Caprolactone) Synthesized by High Internal Phase Emulsion Templating. Polymers 2020, 12, 1849. [Google Scholar] [CrossRef]
- Hernández, A.R.; Contreras, O.C.; Acevedo, J.C.; Moreno, L.G.N. Poly(ε-Caprolactone) Degradation Under Acidic and Alkaline Conditions. Am. J. Polym. Sci. 2013, 3, 70–75. [Google Scholar] [CrossRef]








| Empirical Formula | C58 H106 O21 Ti4 |
|---|---|
| Formula weight | 1331.02 |
| Temperature | 100(2) K |
| Wavelength | 0.85506 Å |
| Crystal system | Monoclinic |
| Space group | P21/n |
| Unit cell dimensions [Å] and [°] | a = 13.092(3) α = 90°. b = 24.028(5) β = 97.09(3)°. c = 22.752(5) γ = 90° |
| Volume [Å3] | 7102(3) |
| Z, calculated density [Mg/m3] | 4, 1.245 |
| Absorption coefficient [mm−1] | 0.777 |
| F(000) | 2840 |
| Crystal size [mm3] | 0.120 × 0.080 × 0.060 |
| Theta range for data collection | 1.489 to 32.304°. |
| Index ranges | −16 ≤ h ≤ 16 −30 ≤ k ≤ 30 −28 ≤ l ≤ 28 |
| Reflections collected/unique | 88738/14372 [R(int) = 0.0320] |
| Completeness to theta = 30.866° | 99.0% |
| Absorption correction | Numerical |
| Max. and min. transmission | 1.000 and 0.231 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 14372/68/833 |
| Goodness-of-fit on F2 | 1.051 |
| Final R indices [I > 2 sigma (I)] | R1 a = 0.0687, wR2 b = 0.2051 |
| R indices (all data) | R1 a = 0.0704, wR2 b = 0.2068 |
| Largest diff. peak and hole | 1.272 and −0.865 e. Å−3 |
| Modes | (1) | |
|---|---|---|
| IR (cm−1) | R (cm−1) | |
| ν(OH) (water molecules) | 3280 (w) | - |
| 3100 (w) | - | |
| ν(OH) (water molecules) | - | 3080 (w) |
| ν(CH) | 3080 (w) | - |
| ν(C=O) (ester group) | 1774 (m) | - |
| 1762 (m) | 1759 (vw) | |
| ν(C=C) | 1605 (w) | 1608 (s) |
| νas(COO) (carboxylate group) | 1589 (m) | - |
| 1550 (s) | 1551 (m) | |
| νs(COO) + ν(CC) (carboxylate group) | 1483 (w) | 1484 (w) |
| 1467 (m) | - | |
| 1459 (w) | 1459 (m) | |
| 1447 (w) | - | |
| - | 1400 (s) | |
| ν(C-O) (ester group) | 1216 (m) | 1225 (w) |
| ν(C-O) (alkoxide group) | 1096 (s) | - |
| ν(COC) (ester group) | 1050 (m) | - |
| ν(C-O) (alkoxide group) | 1026 (s) | - |
| ν(CC) | 753 (m) | 755 (w) |
| νa(Ti-(μ-O)-Ti) | 708 (m) | - |
| 692 (s) | ||
| δ(CH) | 683 (m) | 676 (m) |
| 666 (s) | ||
| νa(Ti-(μ4-O)-Ti) + | 650 (s) | 649 (m) |
| δ(OCO) (ester) | 611 (w) | 600 (w) |
| 583 (m) | ||
| νa(Ti-(μ4-O)-Ti) | 552 (s) | |
| νs(Ti-(μ4-O)-Ti) | 536 (w) | |
| 539 (s) | ||
| 519 (w) | ||
| 513 (vw) | ||
| DSC | TGA | |||
|---|---|---|---|---|
| Composite | Stage | Solid Residue | ||
| Tm/°C | Td/°C | Tdmax/°C | at 450 °C (%) | |
| PCL | 41.2 | 318.2 | 347 | 2.11 |
| PCL + (1) 10 wt.% | 40.3 | 344.6 | 384 | 10.81 |
| PCL + (1) 15 wt.% | 41.8 | 339.8 | 395 | 15.77 |
| PCL + (1) 20 wt.% | 42 | 340.7 | 391 | 18.03 |
| Composite | MB Decolorization (%) |
|---|---|
| MB-irradiated | 33.03 |
| PCL | 37.1 |
| PCL + (1) 10 wt.% | 89.22 |
| PCL + (1) 15 wt.% | 100 |
| PCL + (1) 20 wt.% | 94.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmigiel, J.; Muzioł, T.; Piszczek, P.; Radtke, A. Titanium(IV) Oxo-Complex with Acetylsalicylic Acid Ligand and Its Polymer Composites: Synthesis, Structure, Spectroscopic Characterization, and Photocatalytic Activity. Materials 2022, 15, 4408. https://doi.org/10.3390/ma15134408
Śmigiel J, Muzioł T, Piszczek P, Radtke A. Titanium(IV) Oxo-Complex with Acetylsalicylic Acid Ligand and Its Polymer Composites: Synthesis, Structure, Spectroscopic Characterization, and Photocatalytic Activity. Materials. 2022; 15(13):4408. https://doi.org/10.3390/ma15134408
Chicago/Turabian StyleŚmigiel, Julia, Tadeusz Muzioł, Piotr Piszczek, and Aleksandra Radtke. 2022. "Titanium(IV) Oxo-Complex with Acetylsalicylic Acid Ligand and Its Polymer Composites: Synthesis, Structure, Spectroscopic Characterization, and Photocatalytic Activity" Materials 15, no. 13: 4408. https://doi.org/10.3390/ma15134408
APA StyleŚmigiel, J., Muzioł, T., Piszczek, P., & Radtke, A. (2022). Titanium(IV) Oxo-Complex with Acetylsalicylic Acid Ligand and Its Polymer Composites: Synthesis, Structure, Spectroscopic Characterization, and Photocatalytic Activity. Materials, 15(13), 4408. https://doi.org/10.3390/ma15134408