Biological Graft as an Innovative Biomaterial for Complex Skin Wound Treatment in Dogs: A Preliminary Report
Abstract
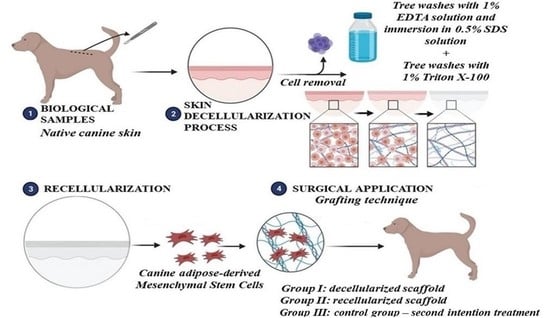
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Skin Fragment Collection
2.3. Skin Decellularization
2.4. Scaffolds’ Histological Analysis
2.5. DAPI Fluorescence
2.6. Scaffolds’ Ultrastructural Analysis
2.7. gDNA Quantification
2.8. Biological Scaffold Recellularization
2.9. Untreated and Treated Animal Groups
2.10. Preoperative and Wound Debridement
2.11. Scaffold Grafting Technique
2.12. Statistical Analysis
3. Results
3.1. Canine Skin Decellularization Analysis
3.2. Genomic DNA Quantification
3.3. Analysis of Recellularized Scaffolds
3.4. Pre- and Post-Grafting Macroscopic Analysis
3.5. Microscopic Analysis
- Histopathological descriptions of Group I (30 days post-grafting)
- Histopathological descriptions of Group I (60 days post-grafting)
- Histopathological descriptions of Group II (30 days post-grafting)
- Histopathological descriptions of Group II (60 days post-grafting)
3.6. Statistical Analysis—Complete Wound Healing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, M.V.B.; Padilha, F.N.; Perugini, M.R.E. Deep tissue culture and hemoculture in dogs with wounds and sepsis. Pesqui. Veterinária Bras. 2017, 37, 1483–1490. [Google Scholar] [CrossRef]
- Swaim, S.F.; Angarano, D.W. Chronic problem wounds of dog limbs. Clin. Dermatol. 1990, 8, 175–186. [Google Scholar] [CrossRef]
- Pavletic, M.M. Atlas of Small Animal Wound Management and Reconstructive Surgery, 4th ed.; John Wiley & Sons: New York, NY, USA, 2018; ISBN 978-1-119-26750-8. [Google Scholar]
- Lopes, M.A.I.; Morais, L.H.S.; Correia, J.H.D. Abordagem e Maneio Médico-Cirúrgico de Feridas Abertas em Cães e Gatos: Caracterização Etiológica e Estudo de Padrões Traumáticos. Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2016. [Google Scholar]
- Amalsadvala, T.; Swaim, S.F. Management of Hard-to-Heal Wounds. Vet. Clin. North Am. Small Anim. Pract. 2006, 36, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Kirpensteijn, J.; ter Haar, G. Reconstructive Surgery and Wound Management of the Dog and Cat (English Edition)-eBooks em Inglês na Amazon.com.br, 1st ed.; CRC Press, Ed.; Manson Publishing/The Veterinary Press: London, UK, 2013; ISBN 1840761636. [Google Scholar]
- Fossum, T.W.; Duprey, L.P. Small Animal Surgery, 5th ed.; Philadelphia, P., Ed.; Elsevier: Philadelphia, PA, USA, 2019; ISBN 9780323443449. [Google Scholar]
- Zubin, E.; Conti, V.; Leonardi, F.; Zanichelli, S.; Ramoni, R.; Grolli, S. Regenerative therapy for the management of a large skin wound in a dog. Clin. Case Rep. 2015, 3, 598. [Google Scholar] [CrossRef]
- Abu-Seida, A.M. Effect of Propolis on Experimental Cutaneous Wound Healing in Dogs. Vet. Med. Int. 2015, 2015, 672643. [Google Scholar] [CrossRef]
- Jee, C.H.; Eom, N.Y.; Jang, H.M.; Jung, H.W.; Choi, E.S.; Won, J.H.; Hong, I.H.; Kang, B.T.; Jeong, D.W.; Jung, D.I. Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs. J. Vet. Sci. 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Reddell, P.; De Ridder, T.R.; Morton, J.M.; Jones, P.D.; Campbell, J.E.; Brown, G.; Johannes, C.M.; Schmidt, P.F.; Gordon, V. Wound formation, wound size, and progression of wound healing after intratumoral treatment of mast cell tumors in dogs with tigilanol tiglate. J. Vet. Intern. Med. 2021, 35, 430–441. [Google Scholar] [CrossRef]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, H.; Lin, S. Chemical approaches to angiogenesis in development and regeneration. Methods Cell Biol. 2016, 134, 369–376. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Thornton, J.F.; Gosman, A.A. Skin grafts and skin substitutes. Sel. Read. Plast. Surg. 2004, 10, 1–24. [Google Scholar]
- Hermans, M.H.E. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: Is there a clinical difference? Burns 2014, 40, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Nyame, T.T.; Chiang, H.A.; Orgill, D.P. Clinical Applications of Skin Substitutes. Surg. Clin. 2014, 94, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Centanni, J.M.; Straseski, J.A.; Wicks, A.; Hank, J.A.; Rasmussen, C.A.; Lokuta, M.A.; Schurr, M.J.; Foster, K.N.; Faucher, L.D.; Caruso, D.M.; et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: Results from a prospective, randomized, controlled dose escalation trial. Ann. Surg. 2011, 253, 683. [Google Scholar] [CrossRef] [PubMed]
- Shores, J.T.; Brandacher, G.; Lee, W.P.A. Hand and upper extremity transplantation: An update of outcomes in the worldwide experience. Plast. Reconstr. Surg. 2015, 135, 351e–360e. [Google Scholar] [CrossRef]
- Jeremias, E.; Bedaiwy, M.A.; Gurunluoglu, R.; Biscotti, C.V.; Siemionow, M.; Falcone, T. Heterotopic autotransplantation of the ovary with microvascular anastomosis: A novel surgical technique. Fertil. Steril. 2002, 77, 1278–1282. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Balestrini, J.L.; Gard, A.L.; Liu, A.; Leiby, K.L.; Schwan, J.; Kunkemoeller, B.; Calle, E.A.; Sivarapatna, A.; Lin, T.; Dimitrievska, S.; et al. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr. Biol. 2015, 7, 1598–1610. [Google Scholar] [CrossRef]
- Sakina, R.; Llucià-Valldeperas, A.; Henriques Lourenço, A.; Harichandan, A.; Gelsomino, S.; Wieringa, P.; Mota, C.; Moroni, L. Decellularization of porcine heart tissue to obtain extracellular matrix based hydrogels. Methods Cell Biol. 2020, 157, 3–21. [Google Scholar] [CrossRef]
- Luo, Y.; Lou, D.; Ma, L.; Gao, C. Optimizing detergent concentration and processing time to balance the decellularization efficiency and properties of bioprosthetic heart valves. J. Biomed. Mater. Res. A 2019, 107, 2235–2243. [Google Scholar] [CrossRef]
- Schallberger, S.P.; Stanley, B.J.; Hauptman, J.G.; Steficek, B.A. Effect of porcine small intestinal submucosa on acute full-thickness wounds in dogs. Vet. Surg. 2008, 37, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsa, I.M.; Culp, W.T.N. Wound Care. Vet. Clin. North Am. Small Anim. Pract. 2015, 45, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 1578. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Nascimento, M.; De Almeida Junior, E.; Crusoé, M.; Bahia, P.; Rosa, F.P. Avanços e aplicações da bioengenharia tecidual. Rev. Ciências Médicas e Biológicas 2010, 9, 28. [Google Scholar] [CrossRef]
- Hopper, R.A.; Woodhouse, K.; Semple, J.L. Acellularization of human placenta with preservation of the basement membrane: A potential matrix for tissue engineering. Ann. Plast. Surg. 2003, 51, 598–602. [Google Scholar] [CrossRef]
- Hubmacher, D.; Apte, S.S. The biology of the extracellular matrix: Novel insights. Curr. Opin. Rheumatol. 2013, 25, 65–70. [Google Scholar] [CrossRef]
- da Palma, R.K.; Fratini, P.; Schiavo Matias, G.S.; Cereta, A.D.; Guimarães, L.L.; Anunciação, A.R.d.A.; de Oliveira, L.V.F.; Farre, R.; Miglino, M.A. Equine lung decellularization: A potential approach for in vitro modeling the role of the extracellular matrix in asthma. J. Tissue Eng. 2018, 9, 2041731418810164. [Google Scholar] [CrossRef]
- Martins, A.R.; Matias, G.S.S.; Batista, V.F.; Miglino, M.A.; Fratini, P. Wistar rat dermis recellularization. Res. Vet. Sci. 2020, 131, 222–231. [Google Scholar] [CrossRef]
- Koga, B.A.A. Effect of Recombinant Human Peptide Growth Factors (PDGF-BB and/or VEGF165) on Mesenchymal Stem Cell Secretoma and on an Animal Model of Skin Healing. Ph.D. Thesis, School of Veterinary Medicine and Animal Science of the University of São Paulo (FMVZ-USP), São Paulo, Brazil, 2021. [Google Scholar] [CrossRef]
- Paim, C.B.V.; Raiser, A.G.; Cardoso, E.; Beck, C. Autologous full thickness mesh skin grafts, to repair wounds in the carpametacarpal regions of dogs: Influence of laser AsGa therapy. Ciência Rural 2002, 32, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Radlinsky, M.; Renberg, W. In vitro comparison of the locking loop and continuous cruciate suture patterns. Vet. Comp. Orthop. Traumatol. 2001, 14, 15–18. [Google Scholar] [CrossRef]
- Slatter, D. Small Animals Surgery: 2 Vol: Slatter, Douglas: Books, 3rd ed.; Saunders: Philadelphia, PA, USA, 2003; ISBN 8520422721. [Google Scholar]
- Bondioli, E.; Purpura, V.; Orlandi, C.; Carboni, A.; Minghetti, P.; Cenacchi, G.; De Luca, G.; Capirossi, D.; Nigrisoli, E.; Melandri, D. The use of an acellular matrix derived from human dermis for the treatment of full-thickness skin wounds. Cell Tissue Bank. 2019, 20, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y. Current status of regenerative medical therapy based on drug delivery technology. Reprod. Biomed. Online 2008, 16, 70–80. [Google Scholar] [CrossRef]
- Brigido, S.A.; Boc, S.F.; Lopez, R.C. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: A pilot study. Orthopedics 2004, 27, S145–S149. [Google Scholar] [CrossRef]
- Mericka, P. Current trends in safety assurance for tissue grafts used in burn treatment. Acta Chir. Plast. 2006, 48, 51–58. [Google Scholar]
- Tognetti, L.; Pianigiani, E.; Ierardi, F.; Mariotti, G.; Perotti, R.; Di Lonardo, A.; Rubegni, P.; Fimiani, M. Current insights into skin banking: Storage, preservation and clinical importance of skin allografts. J. Biorepository Sci. Appl. Med. 2017, 5, 41–56. [Google Scholar] [CrossRef]
- Szentimrey, D. Principles of reconstructive surgery for the tumor patient. Clin. Tech. Small Anim. Pract. 1998, 13, 70–76. [Google Scholar] [CrossRef]
- Daleck, C.R.; Denardi, A.B. Oncologia Em Cães e Gatos, 2nd ed.; Roca: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Oliveira, M.F.; Maia, I.A.; Noritomi, P.Y.; Nargi, G.C.; Silva, J.V.L.; Ferreira, B.M.P.; Duek, E.A.R. Construção de Scaffolds para engenharia tecidual utilizando prototipagem rápida. Matéria 2007, 12, 373–382. [Google Scholar] [CrossRef]
- Craighead, H.G.; James, C.D.; Turner, A.M.P. Chemical and topographical patterning for directed cell attachment. Curr. Opin. Solid State Mater. Sci. 2001, 5, 177–184. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Miranville, A.; Heeschen, C.; Sengenès, C.; Curat, C.A.; Busse, R.; Bouloumié, A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef]
- Sterodimas, A.; De Faria, J.; Nicaretta, B.; Pitanguy, I. Tissue engineering with adipose-derived stem cells (ADSCs): Current and future applications. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1886–1892. [Google Scholar] [CrossRef]
- Sá, P.A. Utilização de Engenharia de Tecidos no Tratamento de Feridas Crônicas. Ph.D. Thesis, Universidade Fernando Pessoa, Porto, Portugal, 2015. [Google Scholar]
- Zahorec, P.; Koller, J.; Danisovic, L.; Bohac, M. Mesenchymal stem cells for chronic wounds therapy. Cell Tissue Bank. 2015, 16, 19–26. [Google Scholar] [CrossRef]
- Buote, N.J. Updates in Wound Management and Dressings. Vet. Clin. North Am. Small Anim. Pract. 2022, 52, 289–315. [Google Scholar] [CrossRef]
- Platt, J.; DiSesa, V.; Gail, D.; Massicot-Fisher, J. Recommendations of the National Heart, Lung, and Blood Institute Heart and Lung Xenotransplantation Working Group. Circulation 2002, 106, 1043–1047. [Google Scholar] [CrossRef]
- Mantovani, F.; Trinchieri, A.; Castelnuovo, C.; Romanò, A.L.; Pisani, E. Reconstructive urethroplasty using porcine acellular matrix. Eur. Urol. 2003, 44, 600–602. [Google Scholar] [CrossRef]
- Ansaloni, L.; Cambrini, P.; Catena, F.; Di Saverio, S.; Gagliardi, S.; Gazzotti, F.; Hodde, J.P.; Metzger, D.W.; D’Alessandro, L.; Pinna, A.D. Immune response to small intestinal submucosa (surgisis) implant in humans: Preliminary observations. J. Investig. Surg. 2007, 20, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W.T.; Diegelmann, R.F. Growth factors in wound healing. Clin. Dermatol. 1994, 12, 157–169. [Google Scholar] [CrossRef]











| Dogs Description | Wound Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Breed | Sex | Age (Years) | Comorbidities | Type | Cause | Wound Location | Measures | |
| Dog 1 (Group I) | Bull Terrier | M | 5 | No | Surgical | Actinic dermatitis | Left metatarsal region | 4.32 × 3.75 cm |
| Dog 2 (Group I) | Mixed breed | M | 8 | No | Bite | Laceration by capybara bite | Right lateral thoracic (costal) region | 7.06 × 6.33 cm |
| Dog 3 (Group I) | Mixed breed | M | 12 | No | Surgical | Neoformation | Right metacarpal region | 12.91 × 4.2 cm |
| Dog 4 (Group II) | Mixed breed | M | 12 | No | Surgical | Neoformation | Metatarsophalangeal region, proximal phalangeal region, and right phalangeal region | 6.06 × 4.0 cm |
| Dog 5 (Group II) | Mixed breed | M | 7 | No | Surgical | Ulcerative dermatitis | Lateral region of left forearm | 7.07 × 3.0 cm |
| Dog 6 (Group II) | Mixed breed | M | 7 | No | Surgical | Hyperplastic dermatitis associated with dermal fibrosis | Metacarpophalangeal region, proximal phalangeal region, proximal interphalangeal region, and left-middle phalangeal region | 4.15 × 4.0 cm |
| Dog 7 (Group III-Control) | Mixed breed | F | 12 | No | Surgical | Neoformation | Right lateral metacarpal region | 2.3 × 1.75 cm |
| Dog 8 (Group III-Control) | Mixed breed | F | 12 | No | Bite | Bite skin laceration | Left plantar metacarpal region | 4.9 × 3.7 cm |
| Dogs | Initial Wound in cm2 (Width × Height) | Time for Complete Healing, Post-Graft Wound (Days) | Average Days, Duncan’s Test (Alpha = 0.05) |
|---|---|---|---|
| Dog 1 (Group I) | 4.32 × 3.75 = 16.2 | 31 | 38.6667 |
| Dog 2 (Group I) | 7.06 × 6.33 = 44.69 | 40 | |
| Dog 3 (Group I) | 12.91 × 4.20 = 54.22 | 45 | |
| Dog 4 (Group II) | 6.06 × 4.00 = 24.24 | 45 | 48.3333 |
| Dog 5 (Group II) | 7.07 × 3.00 = 21.21 | 50 | |
| Dog 6 (Group II) | 4.15 × 4.00 = 16.6 | 50 | |
| Dog 7 (Group III) | 2.30 × 3.75 = 4.02 | 24 | 29.5 |
| Dog 8 (Group III) | 4.90 × 3.70 = 18.13 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dall’Olio, A.J.; Matias, G.d.S.S.; Carreira, A.C.O.; de Carvalho, H.J.C.; van den Broek Campanelli, T.; da Silva, T.S.; da Silva, M.D.; Abreu-Silva, A.L.; Miglino, M.A. Biological Graft as an Innovative Biomaterial for Complex Skin Wound Treatment in Dogs: A Preliminary Report. Materials 2022, 15, 6027. https://doi.org/10.3390/ma15176027
Dall’Olio AJ, Matias GdSS, Carreira ACO, de Carvalho HJC, van den Broek Campanelli T, da Silva TS, da Silva MD, Abreu-Silva AL, Miglino MA. Biological Graft as an Innovative Biomaterial for Complex Skin Wound Treatment in Dogs: A Preliminary Report. Materials. 2022; 15(17):6027. https://doi.org/10.3390/ma15176027
Chicago/Turabian StyleDall’Olio, Adriano Jaskonis, Gustavo de Sá Schiavo Matias, Ana Claudia Oliveira Carreira, Hianka Jasmyne Costa de Carvalho, Thais van den Broek Campanelli, Thamires Santos da Silva, Mônica Duarte da Silva, Ana Lúcia Abreu-Silva, and Maria Angélica Miglino. 2022. "Biological Graft as an Innovative Biomaterial for Complex Skin Wound Treatment in Dogs: A Preliminary Report" Materials 15, no. 17: 6027. https://doi.org/10.3390/ma15176027
APA StyleDall’Olio, A. J., Matias, G. d. S. S., Carreira, A. C. O., de Carvalho, H. J. C., van den Broek Campanelli, T., da Silva, T. S., da Silva, M. D., Abreu-Silva, A. L., & Miglino, M. A. (2022). Biological Graft as an Innovative Biomaterial for Complex Skin Wound Treatment in Dogs: A Preliminary Report. Materials, 15(17), 6027. https://doi.org/10.3390/ma15176027