1. Introduction
High-efficiency and high-power-density electrical machines will play a vital role in decarbonisation efforts and in meeting Net Zero Emission targets across the automotive, aerospace and energy sectors. Electrical machine technology roadmaps published by the Advanced Propulsion Centre (APC) [
1] and Aerospace Technology Institute (ATI) [
2] seek continuous power density of between 9 and 25 kW/kg by 2035. These targets are in stark contrast to the 2–5 kW/kg available at present, representing a significant challenge [
1,
3]. Electrical machine windings rely on highly electrically conductive materials such as copper (Cu) and aluminium (Al) and, while silver (Ag) exhibits higher electrical conductivity, it is typically prohibitive by its relatively high cost [
4]. Step changes in electrical machine power density could be realised by simultaneously improving efficiency through optimised materials, targeted conductor geometry and topology and integrated thermal management strategies. In this regard, Additive Manufacturing (AM) offers unparalleled design freedom, enabling optimum winding geometries that are not feasible through traditional manufacturing techniques [
5]. For AM of metals, the Laser Powder Bed Fusion (L-PBF) process is the most mature and advanced technology and has been widely adopted to fabricate medical, aerospace and automotive metal components [
6,
7]. During L-PBF AM, components are fabricated in a layer-by-layer process through the Selective Laser Melting (SLM) of fine metal powder layers on precedingly melted layers. Therefore, the L-PBF process is highly dependent on the laser and material interaction, which is dictated by the associated laser parameters selected [
8]. Some of the main variables include the metal powder feedstock composition, powder Particle Size Distribution (PSD), laser power, laser scan speed, laser spot size and layer thickness. These in turn effect the phase transformation of the metal, molten metal flow and cooling, laser diffusion, scattering, absorptivity and reflection [
9].
Additionally, laser wavelengths in the infrared range (1060–1090 nm) are commonly used in L-PBF systems due to favourable absorption for common L-PBF metals, such as steels that reflect only ~40% of thermal energy [
10]. However, solid Cu commonly featured in electrical coil windings can reflect up to ~98% of the laser energy at infrared laser wavelengths [
11] and, in powder form, Cu still reflects ~71% of laser energy [
10]. Furthermore, feedstock PSD can affect powder spreading, packing density and laser powder interaction [
12,
13,
14], and laser powder interactions can vary with geometry and height in Z [
8,
15]. Therefore, an understanding of the material–laser interaction to maximise material absorptivity while limiting laser reflectivity is fundamental [
9,
16] for successful L-PBF processing of Cu and Ag [
17,
18]. Previous approaches to mitigate the challenges of L-PBF processing Cu include surface modification of powders [
19], addition of nanoparticular substrates [
20], alloying [
21,
22], using alternative wavelength lasers [
23,
24], higher power lasers and modified scan speeds [
19,
25]. Colopi et al. [
25] have successfully processed high-purity Cu with a final density of ~98% using high-powered (>600 W) infrared lasers. Gargalis et al. [
26] compared the laser energy absorptivity between solid and powder Cu powder (99.9%). It was reported that the energy absorptivity of a 100 µm Cu layer is four times that of a solid Cu sample due to the lower thermal conductivity and less reflective nature of the Cu powder feedstock. However, the research also featured a relatively high laser power (>500 W) and slow scan speeds (~150 mm/s), indicating the requirement for a high energy density. Contrastingly, Silbernagel et al. [
27] used a relatively low-powered laser (200 W) and small laser spot L-PBF system to successfully assess the L-PBF processing of high-purity (99.9%) Cu for electrical applications. Although the maximum density achieved ranged between 80.3–85.8% (dependent on scan strategy), the relatively porous L-PBF Cu had higher electrical conductivity than L-PBF aluminium alloy AlSi10Mg; achieving 54% International Annealed Copper Standard (IACS) in comparison with AlSi10Mg, which achieved 29% IACS [
27].
Adding to the challenge, Cu’s oxidisation tendency can result in the formation of oxide layers on powder feedstock during storage and L-PBF processing [
28,
29]. As such, inert gas powder storage and L-PBF build environment control, such as custom gas mixtures and reducing the L-PBF process oxygen content also offer potential for improved laser processing and component fabrication. However, currently, studies investigating gas mixes and oxygen reduction have primarily focused on L-PBF processing of titanium (Ti) [
30,
31,
32], while investigations of oxygen content for processing Cu have largely been focused on electron beam processing, with little investigation regarding L-PBF [
33,
34].
Due to the increased demand for AM of Cu, the AM industry has developed different variants of Cu for specific applications such as heat exchangers, induction heater coils and electrical windings that offer improved electrical and thermal conductive performance. These variants include high-purity and alloyed Cu with different PSDs and associated L-PBF processing parameters [
35]. Accordingly, this study compares the electrical performance of three high-purity Cu industry variants, namely ‘Carpenter Additive Cu’, ‘EOS Copper Cu’ and ‘EOS CuCP’. Additionally, these variants are also compared with pure Ag and Cu–Ag alloys fabricated by L-PBF to investigate the resulting electrical conductivity performance. The addition of Ag to pure Cu was intended to improve the structural stability and the corrosion resistance of the alloy without hampering the electrical conductivity of the base metal at possible elevated temperatures. The powders were subjected to particle size and morphology analysis prior to sample fabrication. Once fabricated, the electrical performance was measured, followed by Computer Tomography (CT) analysis. The influence of electropolishing on the surface finish was also studied to assess the potential application of electrical insulation coatings, with the effects of polishing being quantified in terms of surface roughness. Additionally, the effect of the type of powder recoating blade and the number of laser exposures was also observed. Finally, proof-of-concept coil fabrication is demonstrated to ascertain the feasibility of L-PBF processing of coil windings and heat exchangers using a 400 W L-PBF system. This study is therefore the first to comparatively report the L-PBF fabrication of high-purity (>99% purity) Cu, Ag and Cu–Ag alloys and their resulting electrical performance.
2. Material and Methods
2.1. Laser Powder Bed Fusion
All samples investigated in this study were fabricated using an industrial-grade L-PBF Additive Manufacturing (AM) system on EOS M290 (EOS GmbH, Krailling, Germany). High-purity (>99%) Ag and Cu powders supplied by Legor Spa, Carpenter Additive and EOS GmbH, respectively, were used as feedstock. The EOS M290 L-PBF AM system features a standard 400 W infrared laser system with 100 µm spot size. The SLM process was carried out in an argon atmosphere at an oxygen content below 0.1%. Following fabrication, all samples were removed from the build platforms using non-contact Electrical Discharge Machining (EDM) (EXCETEK, Taichung, Taiwan).
2.2. Powder Characterisation
Powder morphology and PSD can affect the packing density and flowability of AM powders [
36], subsequently dictating the layer packing and material behaviour during the L-PBF process [
36,
37]. Additionally, the composition and layer packing density can also influence the laser reflectivity and absorptivity at the powder bed [
13]. Previous studies investigating the L-PBF processing of high-purity Cu and Ag have reported the enhancement of mechanical and thermal properties through L-PBF in situ Cu–Ag alloying [
17,
21]. However, the electrical performance of high-purity Cu has seen limited attention, and the electrically conductive properties of L-PBF high-purity Ag and Cu–Ag alloys have yet to be reported. Accordingly, this study considers six feedstock powder compositions as listed in
Table 1. Cu, Ag and Cu–Ag feedstock powders, along with their morphology and Particle Volume Distribution (PVD), were characterised using Scanning Electron Microscopy (SEM) and digital imaging particle analysis techniques. SEM analysis was carried out using a Zeiss EVO50 SEM. A Retsch Technology Camsizer X2 was used to validate the particle morphology, size and shape. The Camsizer X2 utilises dynamic image analysis following ISO 13322-2 [
38], where a high-resolution optical system consisting of digital cameras evaluates precise particle characteristics at a rate of 300 images per second.
2.3. L-PBF Parameters and Sample Manufacture
L-PBF process parameters developed by the authors [
8,
18] in previous studies (listed in
Table 2) were used for initial sample fabrication. L-PBF parameters were kept constant to ensure cause and effect analysis and ensure any resultant variations could be attributed to powder supplier, composition, morphology and PSD.
Considerable research has been conducted regarding L-PBF feedstock [
39,
40] and its influence on L-PBF process parameters [
34,
41,
42] and, as such, studies are emerging reporting optimised parameters for L-PBF processing of high-purity Cu [
19,
25,
27]. However, the process and parameter development usually focus on increasing energy density at the powder bed to mitigate the reflective nature of Cu. Limited investigations have reported the effects of scanning strategies, laser exposure time and powder dosing on the influence of processing Cu. Furthermore, there is no literature available regarding the laser exposure and powder recoating strategies for highly reflective feedstocks such as Ag and Cu–Ag alloys. As such, this study considers the AM powder dosing methodology and laser exposure strategies to ascertain the effects on L-PBF Cu, Ag and Cu–Ag fabrication.
2.4. Annealing Process
Annealing is a heat treatment process which alters the microstructure of a material to optimise the resultant properties. Heat treatment of high-purity L-PBF Cu, Ag and Cu–Ag alloys have seen limited investigation, leaving a gap in knowledge regarding processing feasibility and resultant material properties, particularly in relation to electrical conductivity. However, relatively high Ag content (>29% Ag) in Cu–Ag alloys fabricated through ingot casting have seen investigation and optimum recrystallisation annealing processes have been reported as 30 min at 500 °C [
43]. Subsequent studies have reported this annealing process creates atomic lattice contractions in L-PBF Cu–Ag alloys [
21], which when combined with the Cu–Ag interface interactions can increase strength and thermal diffusivity performance [
21]. Accordingly, all samples fabricated for this study followed the annealing process.
2.5. Surface Roughness
L-PBF allows the fabrication of complex metallic structures not feasible with more traditional manufacturing techniques. However, to take advantage of the desirable benefits, a key requirement of the process is to fabricate near-net-shape components with limited post-processing, such as support removal or finishing. Support removal is time-consuming and can negatively affect the L-PBF component surface, in turn creating crack initiation sites negatively effecting mechanical performance [
44,
45]. Additionally, internal supports can be challenging or impossible to remove dependant on the component geometry and feature dimensions. Furthermore, the layer-by-layer nature of the L-PBF process can result in a staircase step effect, resulting in a relatively rough surface [
45,
46]. While rough surfaces can be desirable for some applications, they are usually undesirable due to crack and failure initiation sites [
47] while increasing surface resistance [
46]. Surface machining is a useful tool in this respect and is the most common method utilised to improve surface finish. However, machining access can be challenging or impossible for complex L-PBF structures and alternative surface finishing techniques are required.
Electropolishing is an electro-chemical process commonly utilised for reductions in surface roughness at the micron scale. Material is removed using a DC power supply by setting up a cell between the copper component (anodically charged) and a pair of chemically inert electrodes (cathodically charged) facing the largest surfaces of the part within a conductive viscous medium (typically acidic in nature). A thin anodic film forms around the copper anode where transfer of metals ions occurs from the highest profile areas of the surface, where current density is greatest, and film is thinnest. Metal ions are oxidised by the applied DC current and encouraged to dissolve into the surrounding medium. As component surface finish can be critical for parts that require additional processes (such as insulation coatings for electrical windings), the samples were subjected to electropolishing regimes with varying times and current densities to assess the potential for surface roughness improvement. The effects of anodic polishing on L-PBF-manufactured high-purity Cu sample surface roughness and electrical performance are analysed experimentally. Two samples were immersed in 1 litre of a proprietary phosphoric/solvent mix solution containing metal cheating agents (Anopol 30). The high-purity Cu samples were subjected to current densities of 0.4 a/cm2 and 0.8 a/cm2, respectively, for a minimum of 10 min using a QPX1200SP rectifier (Aim and Thurlby Thundar Instruments, United Kingdom). The solution was used at ambient temperature with agitation provided by a magnetic stirrer bar at 1000 rpm. Electropolished sample surface roughness was analysed and compared with as-built Cu samples, and surface roughness results are reported using a Keyence VHX-7000 (Keyence Ltd., Milton Keynes, UK) digital microscope.
2.6. Density
Porosity and pore morphology can significantly affect material properties, including mechanical [
18] and electrical performance. For L-PBF unoptimised powder feedstock composition, particle distribution and L-PBF process parameters can negatively affect the resultant density, leading to increased porosity content [
18]. Industrial grinding and polishing techniques combined with digital microscopy and SEM analysis are common characterisation methods for density and porosity evaluation. Although these methods provide an indication of material density and pore characteristics, the volumetric data in relation to distribution throughout the samples are limited. Therefore, for this study, the materials were analysed through Computed Tomography (CT) to characterise the volumetric porosity and density.
CT is a non-destructive analysis technique suitable for porosity characterisation. However, the CT data outputs will be dependent on the scanning and threshold parameters set by the operator and, as such, the CT results generated are best suited for comparative rather than absolute analysis. Qualitative analysis of the fabricated samples was conducted using micro-CT on Nikon XT H 225ST (Nikon Metrology Inc., Castle Donington, UK) and internal cross-sectional analysis, completed using SEM on Zeiss EVO50 (Carl Zeiss AG, Jena, Germany). Samples were CT processed at a magnification factor of 22, whilst a Sn filter of 0.25 mm thickness was used. A potential of 223 kV with a beam current of 40 µA resulted in a voxel size of 9 µm. Circa 3100 projections were collected from each sample and reconstructed using CT Agent 3D pro software. The resultant images were vertically stacked using VG Studio max 3.4 software. Post-processing for 3D visualisation was conducted using Dragonfly (Object Research Systems Inc. version 2022.1) software. The reconstructed CT data were subjected to porosity measurements using native algorithms in the Dragonfly software. A cylindrical Region of Interest (RoI) of 30 mm3 was created in all the samples for a standardised comparative analysis.
2.7. Electrical Conductivity
The electrical conductivity was measured using a small-signal 4-wire Ohm meter (RS Pro RM-805) and via large-signal 4-wire measurement method in the range from 10A to 60A DC (Fluke Norma 4000) in accordance with ASTM B193-16 [
48]. The electrical resistivity was measured in two methods having small and big resistance to test the range of conductivity of the additively manufactured samples. The ends of the samples were manually polished to ensure maximum and uniform contact with the testing probes. From the resistance measurements, the conductivity of the samples was calculated. This measurement is mainly influenced by the surface roughness and density of the sample. The presence of peaks and valleys can disrupt the contact area with the testing probes and pores, and any unfused powders are a clear obstruction to the conduction of electricity.
2.8. Design of the Experiments
The L-PBF process is a combination of over 130 feedstocks and process variables related to powder characteristics, process build environment and laser–material interactions [
16]. While material feedstock and common L-PBF process parameters such as laser power and scan speed have seen much investigation, process variables including laser exposure duration and powder recoating can also affect the density of the fabricated components. However, these variables have seen limited investigation in the current literature. Therefore, this study also includes the effect of these parameters on the resultant electrical conductivity performance. Due to the numerous variables investigated in this study, four experiments (
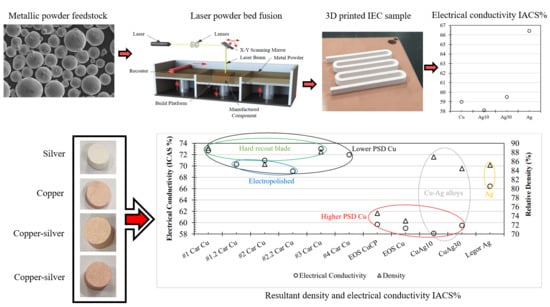
Figure 1) were conducted considering the effects of material composition, powder recoating and laser exposure on electrical performance and electropolishing effects on surface roughness. Material feedstock characteristics were first ascertained, and SEM and CT were utilised to assess fabricated sample density and porosity distribution.
Firstly, three high-purity Cu powders were studied to evaluate the influence of purity on the L-PBF processability using a standard 400 W infrared laser. Secondly, high-purity Cu was compared with high-purity Ag and two Cu–Ag alloys (namely Cu–Ag10% and Cu–Ag20%) to identify the influence of Ag addition on sample density and electrical performance. Thirdly, laser exposure and powder recoating methods were investigated to characterise the effects on resultant density and electrical performance. Finally, the effects of electropolishing regimes on high-purity Cu and the effects on electrical performance were investigated before all results were collated and summarised. A total of nine International Electrotechnical Commission (IEC) samples were fabricated, with two samples further electropolished. While additional process parameter development could be utilised for material optimisation, it was outside the scope of this study.
4. Conclusions
In this work, high-purity copper (Cu), silver (Ag) and copper–silver (Cu–Ag) alloys were additively manufactured using a standard 400 W Laser Powder Bed Fusion (L-PBF) system, and their resultant densities and electrical conductivity performances were evaluated. Six highly reflective and thermally conductive Cu and Ag material variants were comparatively investigated focusing on material purity, Ag content in Cu–Ag alloys, recoating, laser exposure and electropolishing. The results show a link between Cu feedstock characteristics and the resulting electrical conductivity of the L-PBF processed samples. Looking at the influence of Cu purity on electrical performance, no corelation was found, with the highest purity Cu (>99.98%) performing similar to (59.7% and 59% IACS) the lowest purity Cu (>99%). For Cu, higher layer packing density dictated by lower feedstock PSD had a significant positive effect on sample density and electrical performance. When it comes to Cu–Ag, the relative densities and electrical performance were found to be unrelated. This is primarily due to electrical performance being restricted by Cu–Ag interfaces negatively effecting electron conduction through dislocation density and scattering mechanisms. However, it was found that L-PBF process parameters utilised for sample fabrication resulted in denser Ag in comparison with Cu, which consequently resulted in comparatively higher electrical performance. Looking at the impact of powder recoating and laser exposure on L-PBF Cu processing, hard blade recoating and single-laser exposure strategies showed improvements in density, with increases of 2.8% and 2% IACS electrical conductivity for Cu when compared with soft blade recoating and double-laser exposure strategies. Lastly, electropolishing was found to improve the Cu surface roughness from Ra of 6.42 µm to 2.78 µm. Nevertheless, current densities and immersion durations twice that of electropolishing similar copper grades manufactured by other methods are required for L-PBF-processed variants. Overall, the results show that contrary to recent literature and commonly agreed understanding, L-PBF processing of high-purity Cu, Ag and Cu–Ag is feasible using a standard laser 400 W L-PBF AM system. Additionally, further improvements in component density and electrical performance could be achieved through the optimisation of powder feedstock PSD and L-PBF process parameters specifically for Cu 400 W processing.