Characterization of Self-Growing Biomaterials Made of Fungal Mycelium and Various Lignocellulose-Containing Ingredients
Abstract
:1. Introduction
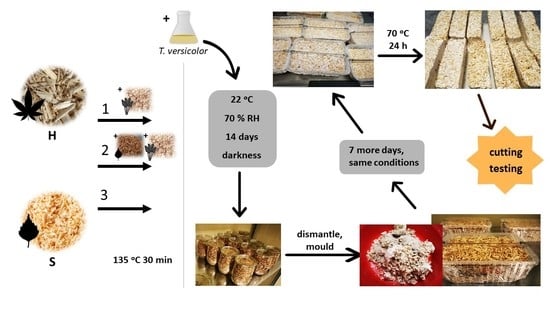
2. Materials and Methods
3. Results and Discussion
3.1. Mycelial Biomass
3.2. Chemical Analyses of MB Components
3.3. Ash Content and Elemental Composition
3.4. Water-Related Properties
3.5. Mechanical Properties
3.6. Susceptibility to Mold Growth
3.7. Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Pellegrini, M.; Savino, E. Physico-Mechanical and Thermodynamic Properties of Mycelium-Based Biocomposites: A Review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Zabel, R.; Morrell, J. Wood Microbiology. Decay and Its Prevention; Academic Press: Cambridge, MA, USA, 2020; p. 576. [Google Scholar]
- Baldrian, P.; Valásková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, G. Chapter 8—Fungal Degradation of Wood Cell Walls. In Secondary Xylem Biology; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 131–167. [Google Scholar] [CrossRef]
- Schmidt, O. Wood and Tree Fungi; Springer: Heidelberg, Germany, 2006. [Google Scholar]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, F.; Zhuo, R.; Fan, F.; Liu, H.; Zhang, C.; Ma, L.; Jiang, M.; Zhang, X. Enhancing the laccase production and laccase gene expression in the white-rot fungus Trametes velutina 5930 with great potential for biotechnological applications by different metal ions and aromatic compounds. PLoS ONE 2013, 8, e79307. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef] [Green Version]
- Irbe, I.; Filipova, I.; Skute, M.; Zajakina, A.; Spunde, K.; Juhna, T. Characterization of Novel Biopolymer Blend Mycocel from Plant Cellulose and Fungal Fibers. Polymers 2021, 13, 1086. [Google Scholar] [CrossRef]
- Pelletier, M.G.; Holt, G.A.; Wanjura, J.D.; Bayer, E.; McIntyre, G. An evaluation study of mycelium based acoustic absorbers grown on agricultural by-product substrates. Ind. Crops Prod. 2013, 51, 480–485. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium Composites: A Review of Engineering Characteristics and Growth Kinetics. J. Bionanosci. 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Chan, W.K. Cost modeling and optimization of a manufacturing system for mycelium-based biocomposite parts. J. Manuf. Syst. 2016, 41, 8–20. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium bio-composites in industrial design and architecture: Comparative review and experimental analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [Green Version]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A comprehensive framework for the production of mycelium-based lignocellulosic composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef]
- Browning, B.L.J. Methods of Wood Chemistry; Wiley & Sons, Interscience Publishers: New York, NY, USA, 1967; Volume I. [Google Scholar]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A.B. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Rizikovs, J.; Paze, A.; Plavniece, A.; Stankus, K.; Virsis, I. A novel method for birch outer bark quality control using higher heating value. In Proceedings of the 11th International Scientific and Practical Conference, Rezekne, Latvia, 21–27 March 2005; pp. 282–285. [Google Scholar]
- Liimatainen, J.; Karonen, M.; Sinkkonen, J.; Helander, M.; Salminen, J.-P. Characterization of phenolic compounds from inner bark of Betula pendula. Holzforschung 2012, 66, 171–181. [Google Scholar] [CrossRef]
- Kumar, A.; Korpinen, R.; Möttönen, V.; Verkasalo, E. Suberin Fatty Acid Hydrolysates from Outer Birch Bark for Hydrophobic Coating on Aspen Wood Surface. Polymers 2022, 14, 832. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Lærke, H.N.; Knudsen, K.-E.B.; Lampi, A.-M.; Piironen, V.; Adlercreutz, H.; Katina, K.; Poutanen, K.; Åman, P. Physical, microscopic and chemical characterisation of industrial rye and wheat brans from the Nordic countries. Food Nutr. Res. 2009, 53, 1912. [Google Scholar] [CrossRef] [Green Version]
- De Souza, D.F.; Tychanowicz, G.K.; de Souza, C.G.; Peralta, R.M. Co-production of ligninolytic enzymes by Pleurotus pulmonarius on wheat bran solid state cultures. J. Basic Microbiol. 2006, 46, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Irbe, I.; Andersone, I.; Andersons, B.; Tsiklauri, N. Hydrolytic enzyme activity of EN113 standard basidiomycetes in the fermentation of lignocellulosic material and wood colonization. Holzforschung 2012, 66, 841–847. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Hess, J.R.; Boardman, R.D.; Wright, C.T.; Westover, T.L. Formulation, Pretreatment, and Densification Options to Improve Biomass Specifications for Co-Firing High Percentages with Coal. Ind. Biotechnol. 2012, 8, 113–132. [Google Scholar] [CrossRef]
- Blondeau, D.; St-Pierre, A.; Bourdeau, N.; Bley, J.; Lajeunesse, A.; Desgagné-Penix, I. Antimicrobial activity and chemical composition of white birch (Betula papyrifera Marshall) bark extracts. MicrobiologyOpen 2020, 9, e00944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostapiuk, A.; Kurach, Ł.; Strzemski, M.; Kurzepa, J.; Hordyjewska, A. Evaluation of Antioxidative Mechanisms In Vitro and Triterpenes Composition of Extracts from Silver Birch (Betula pendula Roth) and Black Birch (Betula obscura Kotula) Barks by FT-IR and HPLC-PDA. Molecules 2021, 26, 4633. [Google Scholar] [CrossRef] [PubMed]
- Irbe, I.; Elisashvili, V.; Asatiani, M.D.; Janberga, A.; Andersone, I.; Andersons, B.; Biziks, V.; Grinins, J. Lignocellulolytic activity of Coniophora puteana and Trametes versicolor in fermentation of wheat bran and decay of hydrothermally modified hardwoods. Int. Biodeterior. Biodegrad. 2014, 86, 71–78. [Google Scholar] [CrossRef]
- Hatakka, A.; Maijala, P.; Mettälä, A.; Hakala, T.; Hauhio, L.; Ellmén, J. Fungi as potential assisting agents in softwood pulping. In Progress in Biotechnology; Viikari, L., Lantto, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 21, pp. 81–88. [Google Scholar]
- Whiteway, M.; Bachewich, C. Fungal Genetics. In Fungi: Biology and Applications, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2017; Chapter 2; pp. 37–66. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties Characterization of Chemically Modified Hemp Hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- Melo, R.R.; Stangerlin, D.M.; Santana, R.R.C.; Pedrosa, T.D. Physical and mechanical properties of particleboard manufactured from wood, bamboo and rice husk. Mater. Res. 2014, 17, 682–686. [Google Scholar] [CrossRef]
- Hýsek, Š.; Sikora, A.; Schönfelder, O.; Böhm, M. Physical and mechanical properties of boards made from modified rapeseed straw particles. BioResources 2018, 13, 6396–6408. [Google Scholar] [CrossRef]
- Jiang, Y.; Lawrence, M.; Ansell, M.P.; Hussain, A. Cell wall microstructure, pore size distribution and absolute density of hemp shiv. R. Soc. Open Sci. 2018, 5, 171945. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Calabria-Holley, J.; Jiang, Y.; Lawrence, M. Modification of hemp shiv properties using water-repellent sol-gel coatings. J. Sol-Gel Sci. Technol. 2018, 86, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Stevulova, N.; Cigasova, J.; Purcz, P.; Schwarzova, I.; Kacik, F.; Geffert, A. Water Absorption Behavior of Hemp Hurds Composites. Materials 2015, 8, 2243–2257. [Google Scholar] [CrossRef] [Green Version]
- Wösten, H.A. Hydrophobins: Multipurpose proteins. Annu. Rev. Microbiol. 2001, 55, 625–646. [Google Scholar] [CrossRef] [Green Version]
- Gozdecki, C.; Wilczyński, A. Effects of Wood Particle Size and test Specimen Size on Mechanical and Water Resistance Properties of Injected Wood-High Density Polyethylene Composite. Wood Fiber Sci. 2015, 47, 365–374. [Google Scholar]
- Bumanis, G.; Irbe, I.; Sinka, M.; Bajare, D. Biodeterioration of Sustainable Hemp Shive Biocomposite Based on Gypsum and Phosphogypsum. J. Nat. Fibers 2021, 1–14. [Google Scholar] [CrossRef]
- Irbe, I.; Grinins, J.; Zimele, Z. Wood composites vs mycelium composites—Water absorption and mould growth properties. In Proceedings of the 9th Hardwood Conference—Part I, Sopron, Hungary, 2020; pp. 121–127. [Google Scholar]
- Hubbe, M.A.; Nazhad, M.; Sánchez, C. Composting as a way to convert cellulosic biomass and organic waste into high-value soil amendments: A review. BioRes 2010, 5, 2808–2854. [Google Scholar] [CrossRef]





| Fraction Size mm | Sawdust wt % | Hemp wt % |
|---|---|---|
| ≥10 | 5 | 8 |
| 7 | 1 | 10 |
| 5 | 4 | 28 |
| 3 | 21 | 37 |
| <3 | 69 | 17 |
| MB Variant | Initial Mycelium % | Final Mycelium % | Initial Fungus:Substrate Ratio | Final Fungus:Substrate Ratio |
|---|---|---|---|---|
| S I (WB, water) | 0.44 | 23.9 | 0.004:1 | 0.30:1 |
| S II (WB, BB, water) | 0.34 | 16.9 | 0.003:1 | 0.20:1 |
| S III (water) | 0.44 | 4.2 | 0.004:1 | 0.04:1 |
| H I (WB, water) | 0.44 | 43.7 | 0.004:1 | 0.80:1 |
| H II (WB, BB, water) | 0.34 | 44 | 0.003:1 | 0.80:1 |
| H III (water) | 0.44 | 42.9 | 0.004:1 | 0.75:1 |
| Ingredient | Cellulose % | Klason Lignin % | Ash % | Other Components ** % |
|---|---|---|---|---|
| S | 48.33 ± 0.20 | 23.39 ± 0.66 | 0.42 ± 0.02 | 27.86 |
| H | 44.95 ± 0.58 | 24.30 ± 0.07 | 1.99 ± 0.02 | 28.76 |
| BB | 15.60 ± 0.90 | 79.00 ± 9.50 * | 0.65 ± 0.12 | 4.73 |
| WB | 12.60 ± 0.40 | 10.28 ± 0.12 | 5.62 ± 0.02 | 71.5 |
| MB Variant | Ash % | Nitrogen % | Carbon % | Hydrogen % |
|---|---|---|---|---|
| S I-0 | 0.75 ± 0.02 | 0.26 ± 0.02 | 47.47 ± 0.15 | 5.98 ± 0.15 |
| S II-0 | 1.10 ± 0.12 | 0.34 ± 0.07 | 52.31 ± 4.12 | 6.41 ± 0.42 |
| S III-0 | 0.42 ± 0.02 | 0.25 ± 0.09 | 47.48 ± 0.08 | 5.89 ± 0.10 |
| S I-14 | 1.35 ± 0.02 | 0.94 ± 0.13 | 46.66 ± 0.47 | 6.03 ± 0.17 |
| S II-14 | 1.67 ± 0.05 | 0.95 ± 0.01 | 51.72 ± 1.87 | 6.44 ± 0.03 |
| S III-14 | 0.57 ± 0.07 | 0.23 ± 0.04 | 46.67 ± 0.12 | 5.92 ± 0.09 |
| S I-21 | 1.64 ± 0.01 | 0.77 ± 0.06 | 46.83 ± 0.08 | 6.00 ± 0.13 |
| S II-21 | 1.94 ± 0.01 | 0.79 ± 0.07 | 56.76 ± 0.95 | 6.83 ± 0.18 |
| S III-21 | 0.65 ± 0.00 | 0.31 ± 0.05 | 47.25 ± 0.17 | 6.15 ± 0.05 |
| H I-0 | 2.53 ± 0.08 | 0.71 ± 0.05 | 46.92 ± 0.35 | 5.93 ± 0.04 |
| H II-0 | 1.70 ± 0.04 | 0.73 ± 0.08 | 51.91 ± 0.92 | 6.44 ± 0.09 |
| H III-0 | 1.99 ± 0.02 | 0.60 ± 0.04 | 46.93 ± 0.22 | 5.98 ± 0.10 |
| H I-14 | 2.67 ± 0.02 | 1.21 ± 0.05 | 46.30 ± 0.22 | 5.98 ± 0.05 |
| H II-14 | 1.98 ± 0.04 | 1.04 ± 0.06 | 47.10 ± 0.55 | 5.93 ± 0.15 |
| H III-14 | 2.41 ± 0.02 | 1.09 ± 0.11 | 45.69 ± 1.18 | 5.75 ± 0.10 |
| H I-21 | 4.40 ± 0.03 | 1.24 ± 0.13 | 45.84 ± 0.32 | 5.89 ± 0.21 |
| H II-21 | 3.72 ± 0.00 | 0.94 ± 0.06 | 54.74 ± 1.96 | 6.32 ± 0.43 |
| H III-21 | 3.89 ± 0.06 | 1.08 ± 0.09 | 46.56 ± 0.27 | 5.89 ± 0.04 |
| MB Variant | Density g cm−3 | σ10 MPa | E MPa |
|---|---|---|---|
| S I-21 | 0.200 ± 0.030 | 0.225 ± 0.028 | 2.910 ± 0.465 |
| S II-21 | 0.215 ± 0.021 | 0.178 ± 0.053 | 2.202 ± 0.841 |
| S III-21 | 0.184 ± 0.032 | 0.029 ± 0.009 | 0.296 ± 0.102 |
| H I-21 | 0.103 ± 0.003 | 0.162 ± 0.020 | 2.412 ± 0.587 |
| H II-21 | 0.134 ± 0.011 | 0.198 ± 0.008 | 2.913 ± 0.210 |
| H III-21 | 0.105 ± 0.009 | 0.190 ± 0.015 | 2.938 ± 0.396 |
| MB Variant | Time (Days)/Evaluation Grade (1–4) | Molds Identified | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 12 | 14 | ||
| S I | 0 | 4 | 4 | 4 | 4 | Rhizopus, Trichoderma, Achremonium |
| S II | 0 | 4 | 4 | 4 | 4 | Rhizopus, Trichoderma, Achremonium |
| S III | 0 | 3 | 3 | 3 | 3 | Rhizopus |
| H I | 0 | 4 | 4 | 4 | 4 | Rhizopus, Trichoderma, Achremonium |
| H II | 0 | 4 | 4 | 4 | 4 | Rhizopus, Trichoderma, Achremonium |
| H III | 0 | 4 | 4 | 4 | 4 | Rhizopus, Trichoderma, Achremonium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irbe, I.; Loris, G.D.; Filipova, I.; Andze, L.; Skute, M. Characterization of Self-Growing Biomaterials Made of Fungal Mycelium and Various Lignocellulose-Containing Ingredients. Materials 2022, 15, 7608. https://doi.org/10.3390/ma15217608
Irbe I, Loris GD, Filipova I, Andze L, Skute M. Characterization of Self-Growing Biomaterials Made of Fungal Mycelium and Various Lignocellulose-Containing Ingredients. Materials. 2022; 15(21):7608. https://doi.org/10.3390/ma15217608
Chicago/Turabian StyleIrbe, Ilze, Gustavs Daniels Loris, Inese Filipova, Laura Andze, and Marite Skute. 2022. "Characterization of Self-Growing Biomaterials Made of Fungal Mycelium and Various Lignocellulose-Containing Ingredients" Materials 15, no. 21: 7608. https://doi.org/10.3390/ma15217608
APA StyleIrbe, I., Loris, G. D., Filipova, I., Andze, L., & Skute, M. (2022). Characterization of Self-Growing Biomaterials Made of Fungal Mycelium and Various Lignocellulose-Containing Ingredients. Materials, 15(21), 7608. https://doi.org/10.3390/ma15217608