The Effect of Silicon Phase Morphology on Microstructure and Properties of AlSi10Mg Alloys Fabricated by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructural Analysis
3.2. Microhardness
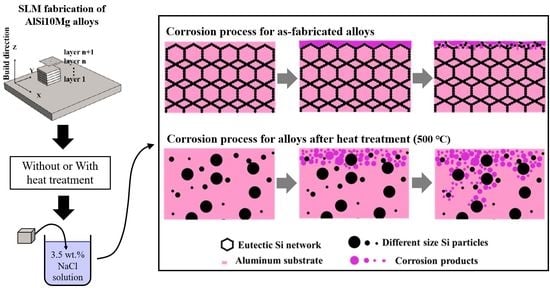
3.3. Corrosion Behavior Analysis
3.3.1. Potentiodynamic Polarization Tests
3.3.2. EIS Measurement
3.3.3. Immersion Test
4. Discussion
4.1. Microstructure Transformation Morphology of Eutectic Silicon
4.2. The Effect of Si Phase Morphology on Mechanical Properties
4.3. The Effect of Si Phase Morphology on Corrosion Behavior
5. Conclusions
- (1)
- The difference in the average grain size and the proportion of large-angle grain boundaries between Alloy A (the SLM directly fabricated AlSi10Mg alloy) and Alloy E (the alloy after heat treatment at 500 °C/5 h) are not distinctive, which indicates that the heat treatment cannot change the grain size and grain boundary distribution of the AlSi10Mg alloys. After heat treatment, the microstructure of the AlSi10Mg alloy is transformed from a continuous network structure with nano-eutectic silicon particles to coarse and homogeneously distributed isolated silicon particles. Moreover, the heat treatment reduces the hardness of AlSi10Mg alloys.
- (2)
- With the coarsening of silicon particles, the corrosion current density increases from 3.39 × 10−7 A·cm−2 to 5.01 × 10−6 A·cm−2, which means the corrosion resistance gradually deteriorates. Based on qualitative and quantitative analysis, the heat treatment reduces the corrosion resistance of SLM-fabricated AlSi10Mg alloys. The corrosion mechanism changes from local pitting to a combination of pitting and intergranular corrosion. Moreover, the directly fabricated alloy corrodes preferentially in the overlapping region of the boundary of the molten pool.
- (3)
- The silicon phase has a dual effect on the corrosion behavior of AlSi10Mg alloys. On one hand, it affects the corrosion driving force on the surface. The electrical potential difference generated by the nano-eutectic silicon particles on the surface is small, which leads to a low chemical reaction activity and an easy formation of a dense protective film. On the other hand, the networked distribution of eutectic silicon benefits the deposition of corrosion products, thereby playing a protective role and slowing down alloy corrosion. With the silicon particles coarsening, the corrosion driving force between the silicon particles and the matrix increases, generating a loose and porous oxide film on the surface. Meanwhile, the corrosion products on the surface of the isolated silicon particles are easily transferred to the solution, which is not conducive to the deposition of corrosion products and weakens the alloy’s corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larráyoz, X.; Blank, A.G.; Pyczak, F. Characterization and modeling of the influence of artificial aging on the microstructural evolution of age-hardenable AlSi10Mg(Cu) aluminum alloys. Mater. Sci. Eng. A 2014, 610, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, J.; Prashanth, K.G.; Scudino, S. Simultaneous enhancements of strength and toughness in an Al-12Si alloy synthesized using selective laser melting. Acta Mater. 2016, 115, 285–294. [Google Scholar] [CrossRef]
- Wu, R.R.; Yuan, Z.; Li, Q.S. Microstructure and mechanical properties of 7075 Al alloy based composites with Al2O3 nanoparticles. Int. J. Cast Met. Res. 2017, 30, 1–4. [Google Scholar]
- Yang, X.; Zhao, Z.Y.; Bai, P.K.; Wang, S.W. EBSD investigation on the microstructure of Ti48Al2Cr2Nb alloy hot isostatic pressing formed by selective laser melting. Mater. Lett. 2021, 309, 131334. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, P.; Misra, R. AlSi10Mg alloy nanocomposites reinforced with aluminum-coated graphene: Selective laser melting, interfacial microstructure and property analysis. J. Alloys Compd. 2019, 792, 203–214. [Google Scholar] [CrossRef]
- Zhuo, L.; Wang, Z.; Zhang, H. Effect of post-process heat treatment on microstructure and properties of selective laser melted AlSi10Mg alloy. Mater. Lett. 2018, 234, 196–200. [Google Scholar] [CrossRef]
- Takata, N.; Kodaira, H.; Sekizawa, K. Change in microstructure of selectively laser melted AlSi10Mg alloy with heat treatments. Mater. Sci. Eng. A 2017, 704, 218–228. [Google Scholar] [CrossRef]
- Wei, L.; Shuai, L.; Jie, L. Effect of heat treatment on AlSi10Mg alloy fabricated by selective laser melting: Microstructure evolution, mechanical properties and fracture mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar]
- Fiocchi, J.; Tuissi, A.; Bassani, P. Low temperature annealing dedicated to AlSi10Mg selective laser melting products. J. Alloys Compd. 2017, 695, 3402–3409. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Maskery, I.; Tuck, C. The microstructure and mechanical properties of selectively laser melted AlSi10Mg: The effect of a conventional T6-like heat treatment. Mater. Sci. Eng. A 2016, 667, 139–146. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, Y.; Lu, R. Effect of the morphology of long-period stacking ordered phase on mechanical properties and corrosion behavior of cast Mg-Zn-Y-Ti alloy. J. Alloys Compd. 2018, 764, 226–238. [Google Scholar]
- Zhang, X.; Zhou, X.; Nilsson, J.O. Corrosion Behaviour of AA6082 Al-Mg-Si Alloy Extrusion: The Influence of Quench Cooling Rate. Corros. Sci. 2019, 15, 100–109. [Google Scholar] [CrossRef]
- Avi, L.; Amnon, S.; Eli, A. Corrosion Behavior of AlSi10Mg Alloy Produced by Additive Manufacturing (AM) vs. Its Counterpart Gravity Cast Alloy. Metals 2016, 6, 148–157. [Google Scholar]
- Yang, C.; Zhang, J.; Gu, X. Distinction of corrosion resistance of selective laser melted Al-12Si alloy on different planes. J. Alloys Compd. 2018, 747, 648–658. [Google Scholar]
- Yan, Y.; Yang, C.; Zhang, J. Improved corrosion behavior of ultrafine-grained eutectic Al-12Si alloy produced by selective laser melting. Mater. Des. 2018, 146, 239–248. [Google Scholar]
- Revilla, R.I.; Liang, J.; Godet, S. Local Corrosion Behavior of Additive Manufactured AlSiMg Alloy Assessed by SEM and SKPFM. J. Electrochem. Soc. 2017, 164, C27–C35. [Google Scholar] [CrossRef]
- Alghamdi, F.; Song, X.; Hadadzadeh, A. Post heat treatment of additive manufactured AlSi10Mg: On silicon morphology, texture and small-scale properties. Mater. Sci. Eng. A 2020, 783, 139296. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, X.J.; Saunders, M. A selective laser melting and solution heat treatment refined Al–12Si alloy with a controllable ultrafine eutectic microstructure and 25% tensile ductility. Acta Mater. 2015, 95, 74–82. [Google Scholar] [CrossRef]
- Wang, L.F.; Sun, J.; Yu, X.L. Enhancement in mechanical properties of selectively laser-melted AlSi10Mg aluminum alloys by T6-like heat treatment. Mater. Sci. Eng. A 2018, 734, 299–310. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Vrancken, B.; Kruth, J.P.; Luyten, J.; Humbeeck, J. Texture and anisotropy in selective laser melting of NiTi alloy. Mater. Sci. Eng. A 2016, 650, 225–232. [Google Scholar] [CrossRef]
- Cizek, P. The microstructure evolution and softening processes during high-temperature deformation of a 21Cr–10Ni–3Mo duplex stainless steel. Acta Mater. 2016, 106, 129–143. [Google Scholar] [CrossRef]
- Lim, S.C.V.; Yang, K.V.; Yang, Y.; Cheng, Y.; Huang, A.; Wu, X.; Davies, C.H.J. Tracking microstructure, texture and boundary misorientation evolution of hot deformed and post-deformation annealed Ti–6Al–4V alloy. Mater. Sci. Eng. A 2016, 651, 524–534. [Google Scholar] [CrossRef]
- Li, M.; Wu, X.; Yang, Y.; Wei, Q.; Yan, C.; Cai, C.; Liu, J.; Li, W.; Shi, Y. TiAl/RGO (reduced graphene oxide) bulk composites with refined microstructure and enhanced nano-hardness fabricated by selective laser melting (SLM). Mater. Charact. 2018, 143, 197–205. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, C.; Liu, Y.; Yang, K.; Lin, J. Characterization of lattice defects and tensile deformation of biomedical Co29Cr9W3Cu alloy produced by selective laser melting. Addit. Manuf. 2019, 30, 100908. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.B.; Zhao, Z.Y.; Li, X.F.; Liu, B.; Bai, P.K. Effect of chloride ion concentration on corrosion process of selective laser melted AlSi10Mg with different heat treatments studied by electrochemical noise. J. Mater. Res. Technol. 2022, 16, 1597–1609. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenz, S.; Pastore, T. Effect of heat treatment on corrosion resistance of DMLS AlSi10Mg alloy. Electrochim. Acta 2016, 206, 346–355. [Google Scholar] [CrossRef]
- Brito, C.; Vida, T.; Freitas, E. Cellular/dendritic arrays and intermetallic phases affecting corrosion and mechanical resistances of an Al-Mg-Si alloy. J. Alloys Compd. 2016, 673, 220–230. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Zhang, D. Electrochemical and DFT studies of quinoline derivatives on corrosion inhibition of AA5052 aluminum alloy in NaCl solution. Appl. Surf. Sci. 2015, 357, 2176–2183. [Google Scholar] [CrossRef]
- Nie, M.; Wang, C.T.; Qu, M. The corrosion behavior of commercial purity titanium processed by high-pressure torsion. J. Mater. Sci. 2014, 49, 2824–2831. [Google Scholar] [CrossRef]
- Li, R.X.; Li, R.D.; Zhao, Y.H. Effect of heat treatment on eutectic silicon morphology and mechanical property of Al-Si-Cu-Mg cast alloys. Trans. Nonferrous Met. Soc. China 2004, 14, 496–500. [Google Scholar]
- Mamizadeh, L.; Sarabi, A.A. Optimization of coating process parameters and surface characterization for vanadium-based conversion coating on 2024 aluminum alloy. Prog. Org. Coat. 2019, 133, 33–43. [Google Scholar] [CrossRef]
- Cao, Z.; Kong, G.; Che, C. Influence of Nd addition on the corrosion behavior of Zn-5%Al alloy in 3.5wt.% NaCl solution. Appl. Surf. Sci. 2017, 426, 67–76. [Google Scholar] [CrossRef]
- Hadadzadeh, A.; Amirkhiz, B.S.; Shakerin, S. Microstructural investigation and mechanical behavior of a two-material component fabricated through selective laser melting of AlSi10Mg on an Al-Cu-Ni-Fe-Mg cast alloy substrate. Addit. Manuf. 2020, 31, 100937. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S. Additive manufacturing of metallic components-Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Rakesh, S.C.; Raja, A.; Nadig, P. Influence of working environment and built orientation on the tensile properties of selective laser melted AlSi10Mg alloy. Mater. Sci. Eng. A 2019, 750, 141–151. [Google Scholar]
- Thijs, L.; Kempen, K.; Kruth, J.P. Fine-structured aluminum products with controllable texture by selective laser melting of pre-alloyed AlSi10Mg powder. Acta Mater. 2013, 61, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Yin, X.; Wang, Y.H. Coating diamond surfaces in a Ti/Si/carbon black/diamond system via thermal explosion. Diam. Relat. Mater. 2022, 127, 109195. [Google Scholar] [CrossRef]
- Hu, Z.P.; Jiang, J.; Liao, F. Kinetics of Precipitation and Coarsening of Si-containing Phases in a SupersaturatedAl-20% Si Alloy. Chin. J. Mater. Res. 2018, 32, 25–32. [Google Scholar]
- Yu, X.F.; Zhang, G.Z.; Wang, X.Y.; Gao, Y.Y.; Jia, G.L.; Hao, Z.Y. Non-equilibrium microstructure of hyper-eutectic Al-Si alloy solidified under superhigh pressure. J. Mater. Sci. 1999, 34, 4149–4152. [Google Scholar] [CrossRef]
- Varga, B.; Fazakas, E.; Hargitai, H. Dilatometer study of rapidly solidified aluminium-silicon based alloys. J. Phys. Conf. Ser. 2009, 144, 012105. [Google Scholar] [CrossRef]
- Matyja, H.; Russell, K.C.; Giessen, B.C. Precipitation of silicon from splat-cooled Al-Si alloys. Metall. Mater. Trans. A 1976, 6, 2249–2252. [Google Scholar] [CrossRef]
- Srinivasan, A.; Huang, Y.; Mendis, C.L. Investigations on microstructures, mechanical and corrosion properties of Mg–Gd–Zn alloys. Mater. Sci. Eng. A 2014, 595, 224–234. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Fan, X. Abnormal corrosion behavior of selective laser melted AlSi10Mg alloy induced by heat treatment at 300 °C. J. Alloys Compd. 2019, 803, 314–324. [Google Scholar] [CrossRef]
- Cao, F.H.; Zhang, Z.; Li, J.F. Corrosion behavior of two heat treatment Al-Zn-Mg-Cu alloys in different intergranular corrosion solution. Trans. Nonferrous Met. Soc. China 2004, 14, 619–625. [Google Scholar]












| Si | Mg | Cu | Ni | Fe | Mn | Ti | Al |
|---|---|---|---|---|---|---|---|
| 10 | 0.4 | <0.25 | <0.05 | <0.25 | <0.1 | <0.15 | Bal. |
| Process Parameter | Laser Power | Exposure Time | Spot Size | Layer Thickness | Point Distance | Atmosphere |
|---|---|---|---|---|---|---|
| Value | 300 W | 140 μs | 70 μm | 30 μm | 0.3 | Argon |
| Alloys | Heat Treatment Process |
|---|---|
| Alloy A | As-fabricated SLM |
| Alloy B | 500 °C × 15 min |
| Alloy C | 500 °C × 1 h |
| Alloy D | 500 °C × 2 h |
| Alloy E | 500 °C × 5 h |
| Point No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Al | 84.11 | 16.98 | 13.10 | 13.00 | 10.20 |
| Si | 15.89 | 83.02 | 86.90 | 87.00 | 89.80 |
| Sample | Icorr (A·cm−2) | Ecorr (V) |
|---|---|---|
| Alloy A | 3.39 × 10−7 | −0.81 |
| Alloy B | 6.92 × 10−7 | −0.88 |
| Alloy C | 1.58 × 10−6 | −0.90 |
| Alloy D | 2.51 × 10−6 | −0.83 |
| Alloy E | 5.01 × 10−6 | −0.91 |
| Samples | Rf (kΩ·cm2) | CPEf × 10−5 (S·sn) | n | Rt (kΩ·cm2) | CPEt × 10−4 (S·sn) | n | W × 10−2 (S·s0.5) | Chi-Square |
|---|---|---|---|---|---|---|---|---|
| Alloy A | 15.70 | 1.41 | 0.82 | 45.12 | 8.14 | 0.80 | 4.35 | 1.70 × 10−3 |
| Alloy B | 7.62 | 2.43 | 0.67 | 9.11 | 3.03 | 1 | 17.59 | 6.32 × 10−3 |
| Alloy C | 1.98 | 1.51 | 0.82 | 3.05 | 3.06 | 0.47 | 5.07 | 4.46 × 10−3 |
| Alloy D | 1.69 | 6.89 | 0.64 | 2.35 | 1.73 | 0.78 | 30.19 | 2.50 × 10−3 |
| Alloy E | 1.11 | 6.69 | 0.67 | 2.07 | 1.89 | 0.77 | 0.45 | 2.49 × 10−3 |
| Atoms | Position A | Position B | Position C | Position D | Position E |
|---|---|---|---|---|---|
| Al | 57.4 | 76.1 | 62.5 | 55.0 | 71.4 |
| O | 33.6 | 13.2 | 26.4 | 34.1 | 19.0 |
| Si | 8.7 | 8.6 | 8.8 | 7.7 | 8.8 |
| Cl | 0.4 | 2.1 | 2.2 | 3.2 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Zhao, Z.; Bai, P.; Zhang, Z.; Li, Y.; Liang, M.; Du, W. The Effect of Silicon Phase Morphology on Microstructure and Properties of AlSi10Mg Alloys Fabricated by Selective Laser Melting. Materials 2022, 15, 8786. https://doi.org/10.3390/ma15248786
Wu L, Zhao Z, Bai P, Zhang Z, Li Y, Liang M, Du W. The Effect of Silicon Phase Morphology on Microstructure and Properties of AlSi10Mg Alloys Fabricated by Selective Laser Melting. Materials. 2022; 15(24):8786. https://doi.org/10.3390/ma15248786
Chicago/Turabian StyleWu, Liyun, Zhanyong Zhao, Peikang Bai, Zhen Zhang, Yuxin Li, Minjie Liang, and Wenbo Du. 2022. "The Effect of Silicon Phase Morphology on Microstructure and Properties of AlSi10Mg Alloys Fabricated by Selective Laser Melting" Materials 15, no. 24: 8786. https://doi.org/10.3390/ma15248786
APA StyleWu, L., Zhao, Z., Bai, P., Zhang, Z., Li, Y., Liang, M., & Du, W. (2022). The Effect of Silicon Phase Morphology on Microstructure and Properties of AlSi10Mg Alloys Fabricated by Selective Laser Melting. Materials, 15(24), 8786. https://doi.org/10.3390/ma15248786