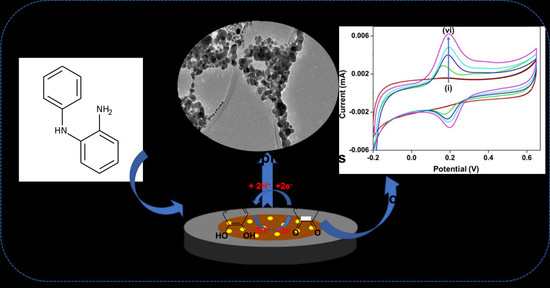
Role of Silver Nanoparticle-Doped 2-Aminodiphenylamine Polymeric Material in the Detection of Dopamine (DA) with Uric Acid Interference
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Electrode Preparation
2.3. Electrochemical Analysis
2.4. Material Preparation
2.5. Material Characterization
3. Results and Discussions
3.1. Material Characterization
3.2. Electrochemical Studies
3.2.1. Cyclic Voltammetry (CV)
3.2.2. CV Study Employing the P-ADPA-Doped Electrode
3.2.3. CV Study Utilizing the AgNPs-2ADPA Doped Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benes, F.M. Carlsson and the discovery of dopamine. Trends Pharmacol. Sci. 2001, 22, 46–47. [Google Scholar] [CrossRef]
- Phillips, P.E.M.; Stuber, G.D.; Heien, M.L.A.V.; Wightman, R.M.; Carelli, R.M. Subsecond dopamine release promotes cocaine seeking. Nature 2003, 422, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Quan, D.P.; Tuyen, D.P.; Lam, T.D.; Tram, P.T.N.; Binh, N.H.; Viet, P.H. Electrochemically selective determination of dopamine in the presence of ascorbic and uric acids on the surface of the modified Nafion/single wall carbon nanotube/poly(3-methylthiophene) glassy carbon electrodes. Colloids Surf. B 2011, 88, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor nanoengineering: Design, operation, and implementation for biomolecular analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Robinson, D.L.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring Rapid Chemical Communication in the Brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Zhu, D.Z.; Gan, H.; Yao, Z.; Fu, Q.; Zhang, X. Prospects and challenges of using electrochemical immunosensors as an alternative detection method for SARS-CoV-2 wastewater-based epidemiology. Sci. Total Environ. 2021, 777, 146239. [Google Scholar] [CrossRef]
- Zhang, C.; You, X.; Li, Y.; Zuo, Y.; Wang, W.; Li, D.; Huang, S.; Hu, H.; Yuan, F.; Shao, F.; et al. A novel electrochemical aptasensor for serum dopamine detection based on methylene blue-integrated m-PdNFs signal material. Sens. Actuators B Chem. 2021, 354, 131233. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and sensors for dopamine detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- Cho, Y.-W.; Park, J.-H.; Lee, K.-H.; Lee, T.; Luo, Z.; Kim, T.-H. Recent advances in nanomaterial-modified electrical platforms for the detection of dopamine in living cells. Nano Converg. 2020, 7, 40. [Google Scholar] [CrossRef]
- Kou, R.; Kobayashi, Y.; Inoue, S.; Tsuchizawa, T.; Ueno, Y.; Suzuki, S.; Hibino, H.; Yamamoto, T.; Nakajima, H.; Yamada, K. Dopamine detection on activated reaction field consisting of graphene-integrated silicon photonic cavity. Opt. Express 2019, 27, 32058–32068. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Dutta Gupta, S. Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J. Hazard. Mater. 2017, 330, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Sookhakian, M.; Basirun, W.J.; Goh, B.T.; Woi, P.M.; Alias, Y. Molybdenum disulfide nanosheet decorated with silver nanoparticles for selective detection of dopamine. Colloids Surf. B 2019, 176, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.; Shukla, S.K.; Islam, R.U.; Witcomb, M.J.; Holzapfel, C.W.; Mallick, K. Polymerization assisted reduction reaction: A sequential electron–proton transfer reaction catalyzed by gold nanoparticle. J. Phys. Chem. C 2013, 117, 23009–23016. [Google Scholar] [CrossRef]
- Fabregat, G.; Córdova-Mateo, E.; Armelin, E.; Bertran, O.; Alemán, C. Ultrathin films of polypyrrole derivatives for dopamine detection. J. Phys. Chem. C 2011, 115, 14933–14941. [Google Scholar] [CrossRef]
- Cui, L. Graphitic carbon nitride sputtered with silver nanoparticles for efficient photocatalytic degradation of rhodamine B dye. Int. J. Electrochem. Sci. 2018, 13, 4981–4990. [Google Scholar] [CrossRef]
- Choudhary, M.; Siwal, S.; Ul Islam, R.; Witcomb, M.J.; Mallick, K. Polymer stabilized silver nanoparticle: An efficient catalyst for proton-coupled electron transfer reaction and the electrochemical recognition of biomolecule. Chem. Phys. Lett. 2014, 608, 145–151. [Google Scholar] [CrossRef]
- Choudhary, M.; Siwal, S.; Nandi, D.; Mallick, K. Single step synthesis of gold–amino acid composite, with the evidence of the catalytic hydrogen atom transfer (HAT) reaction, for the electrochemical recognition of Serotonin. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 77, 72–80. [Google Scholar] [CrossRef]
- Rezazadeh, N.H.; Buazar, F.; Matroodi, S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci. Rep. 2020, 10, 19615. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. Electrochemical sensor for simultaneous determination of epinephrine and norepinephrine based on cetyltrimethylammonium bromide assisted SnO2 nanoparticles. J. Electroanalyt. Chem. 2017, 801, 503–510. [Google Scholar] [CrossRef]
- Siwal, S.; Choudhary, M.; Mpelane, S.; Brink, R.; Mallick, K. Single step synthesis of a polymer supported palladium composite: A potential anode catalyst for the application of methanol oxidation. RSC Adv. 2016, 6, 47212–47219. [Google Scholar] [CrossRef]
- Severinghaus, J.W. First electrodes for blood Po2 and Pco2 determination. J. Appl. Physiol. 2004, 97, 1599–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.T.; Park, I.; Heiranian, M.; Taqieddin, A.; You, S.; Faramarzi, V.; Pak, A.A.; van der Zande, A.M.; Aluru, N.R.; Bashir, R. Ultrasensitive detection of dopamine, IL-6 and SARS-CoV-2 proteins on crumpled graphene FET biosensor. Adv. Mater. Technol. 2021, 6, 2100712. [Google Scholar] [CrossRef]
- Henarejos-Escudero, P.; Contreras-Llano, L.E.; Lozada-Ramírez, J.D.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. A dopamine-based biosynthetic pathway produces decarboxylated betalains in Chenopodium quinoa. Plant Physiol. 2021, 186, 1473–1486. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Kan, X. Electrochemical chiral recognition of tryptophan enantiomers based on copper-modified β-cyclodextrin. J. Electroanalyt. Chem. 2021, 902, 115817. [Google Scholar] [CrossRef]
- Da Silva, L.M.; De Faria, L.A.; Boodts, J.F.C. Electrochemical ozone production: Influence of the supporting electrolyte on kinetics and current efficiency. Electrochim. Acta 2003, 48, 699–709. [Google Scholar] [CrossRef]
- Han, G.; Cai, J.; Liu, C.; Ren, J.; Wang, X.; Yang, J.; Wang, X. Highly sensitive electrochemical sensor based on xylan-based Ag@CQDs-rGO nanocomposite for dopamine detection. Appl. Surf. Sci. 2021, 541, 148566. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Ahmed, F.; Chen, S.-M.; Chen, T.-W.; Hasan, P.M.Z.; Bilgrami, A.L.; Darwesh, R. Rational confinement of yttrium vanadate within three-dimensional graphene aerogel: Electrochemical analysis of monoamine neurotransmitter (dopamine). ACS Appl. Mater. Interfaces 2021, 13, 10987–10995. [Google Scholar] [CrossRef]
- Wang, M.; Guo, H.; Wu, N.; Zhang, J.; Zhang, T.; Liu, B.; Pan, Z.; Peng, L.; Yang, W. A novel triazine-based covalent organic framework combined with AuNPs and reduced graphene oxide as an electrochemical sensing platform for the simultaneous detection of uric acid, dopamine and ascorbic acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127928. [Google Scholar] [CrossRef]
- Patella, B.; Sortino, A.; Mazzara, F.; Aiello, G.; Drago, G.; Torino, C.; Vilasi, A.; O’Riordan, A.; Inguanta, R. Electrochemical detection of dopamine with negligible interference from ascorbic and uric acid by means of reduced graphene oxide and metals-NPs based electrodes. Anal. Chim. Acta 2021, 1187, 339124. [Google Scholar] [CrossRef] [PubMed]
- Brink, R.; Choudhary, M.; Siwal, S.; Nandi, D.; Mallick, K. Silver-polymer functional-nanocomposite: A single step synthesis approach with in-situ optical study. Appl. Surf. Sci. 2017, 412, 482–488. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; Sheoran, K.; Siwal, S.S.; Saini, R.V.; Saini, A.K.; Alsanie, W.F.; Thakur, V.K. Role of Silver Nanoparticle-Doped 2-Aminodiphenylamine Polymeric Material in the Detection of Dopamine (DA) with Uric Acid Interference. Materials 2022, 15, 1308. https://doi.org/10.3390/ma15041308
Kaur H, Sheoran K, Siwal SS, Saini RV, Saini AK, Alsanie WF, Thakur VK. Role of Silver Nanoparticle-Doped 2-Aminodiphenylamine Polymeric Material in the Detection of Dopamine (DA) with Uric Acid Interference. Materials. 2022; 15(4):1308. https://doi.org/10.3390/ma15041308
Chicago/Turabian StyleKaur, Harjot, Karamveer Sheoran, Samarjeet Singh Siwal, Reena V. Saini, Adesh Kumar Saini, Walaa F. Alsanie, and Vijay Kumar Thakur. 2022. "Role of Silver Nanoparticle-Doped 2-Aminodiphenylamine Polymeric Material in the Detection of Dopamine (DA) with Uric Acid Interference" Materials 15, no. 4: 1308. https://doi.org/10.3390/ma15041308
APA StyleKaur, H., Sheoran, K., Siwal, S. S., Saini, R. V., Saini, A. K., Alsanie, W. F., & Thakur, V. K. (2022). Role of Silver Nanoparticle-Doped 2-Aminodiphenylamine Polymeric Material in the Detection of Dopamine (DA) with Uric Acid Interference. Materials, 15(4), 1308. https://doi.org/10.3390/ma15041308