Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Bio-Fabrication Method
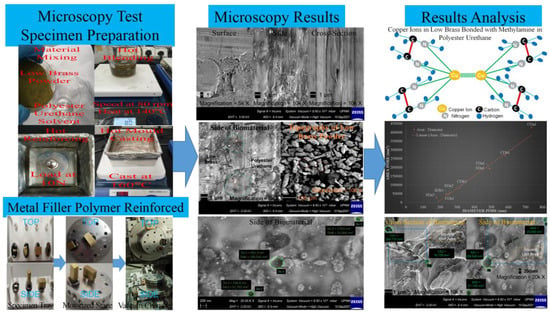
2.3. Test Specimen Preparation
2.4. Microscopy Test Configurations
2.5. Microscopy Tests
3. Results and Discussion
3.1. Membrane Morphology Analysis
3.2. Composition Structure Analysis
3.3. Chemical Characterizations Analysis for Low Brass and Polyester Urethane
3.4. Pore Size Analysis
3.5. Pore Distribution and Correlation Analyses
3.6. Determination of Porosity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–98. [Google Scholar]
- Abdollahiyan, P.; Oroojalian, F.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Nanotechnology, and scaffold implantation for the effective repair of injured organs: An overview on hard tissue engineering. J. Control. Release 2021, 333, 391–417. [Google Scholar] [CrossRef] [PubMed]
- Chinta, M.L.; Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Parcha, S.R. Assessment of properties, applications and limitations of scaffolds based on cellulose and its derivatives for cartilage tissue engineering: A review. Int. J. Biol. Macromol. 2021, 175, 495–515. [Google Scholar] [CrossRef]
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Health Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Tognolo, L.; Maccarone, M.C.; de Trane, S.; Scanu, A.; Masiero, S.; Fiore, P. Therapeutic Exercise and Conservative Injection Treatment for Early Knee Osteoarthritis in Athletes: A Scoping Review. Medicina 2022, 58, 69. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Rux, D.; Helbig, K.; Han, B.; Cortese, C.; Koyama, E.; Han, L.; Pacifici, M. Primary cilia direct murine articular cartilage tidemark patterning through Hedgehog signaling and ambulatory load. J. Bone Miner. Res. 2022. early view. [Google Scholar] [CrossRef] [PubMed]
- Daou, F.; Cochis, A.; Leigheb, M.; Rimondini, L. Current Advances in the Regeneration of Degenerated Articular Cartilage: A Literature Review on Tissue Engineering and Its Recent Clinical Translation. Materials 2021, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Muthaiah, V.M.S.; Indrakumar, S.; Suwas, S.; Chatterjee, K. Surface engineering of additively manufactured titanium alloys for enhanced clinical performance of biomedical implants: A review of recent developments. Bioprinting 2022, 25, e00180. [Google Scholar] [CrossRef]
- Arifvianto, B.; Zhou, J. Fabrication of Metallic Biomedical Scaffolds with the Space Holder Method: A Review. Materials 2014, 7, 3588–3622. [Google Scholar] [CrossRef] [Green Version]
- Zerankeshi, M.M.; Bakhshi, R.; Alizadeh, R. Polymer/metal composite 3D porous bone tissue engineering scaffolds fabricated by additive manufacturing techniques: A review. Bioprinting 2022, 25, e00191. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-Based Nanofiber–Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers 2021, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Jouyandeh, M.; Vahabi, H.; Rabiee, N.; Rabiee, M.; Bagherzadeh, M.; Saeb, M.R. Green composites in bone tissue engineering. Emergent Mater. 2021. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Liu, A.; Pan, C.; Ding, H.; Ye, W. Reduced graphene oxide/titanium dioxide hybrid nanofiller-reinforced electrospun silk fibroin scaffolds for tissue engineering. Mater. Lett. 2021, 291, 129563. [Google Scholar] [CrossRef]
- Qian, H.; Lei, T.; Lei, P.; Hu, Y. Additively Manufactured Tantalum Implants for Repairing Bone Defects: A Systematic Review. Tissue Eng. Part B Rev. 2021, 27, 166–180. [Google Scholar] [CrossRef]
- Wei, F.; Powers, M.J.F.; Narez, G.E.; Dejardin, L.M.; Donahue, T.H.; Haut, R.C. Post-traumatic Osteoarthritis in Rabbits Following Traumatic Injury and Surgical Reconstruction of the Knee. Ann. Biomed. Eng. 2022, 50, 169–182. [Google Scholar] [CrossRef]
- Yik, L.Y.; Miskon, A.; Zaidi, A.M.A.; Ahmad, M.M.H.M.; Bakar, M.A. Mechanical and Structural Strengths Analyses of Low Brass Filler Biomaterial for Articular Cartilage Scaffold Applications. J. Funct. Biomater. 2022; resubmitted. [Google Scholar]
- Ashkarran, A.A.; Hamidinezhad, H.; Haddadi, H.; Mahmoudi, M. Double-doped TiO2 nanoparticles as an efficient visible-light-active photocatalyst and antibacterial agent under solar simulated light. Appl. Surf. Sci. 2014, 301, 338–345. [Google Scholar] [CrossRef]
- Lubit, E.B. Permeability, Osmosis, and Edema. Anesthesiology 2010, 113, 1250–1251. [Google Scholar] [CrossRef] [Green Version]
- Chen, G. Donnan Equilibrium Revisited: Coupling between Ion Concentrations, Osmotic Pressure, and Donnan Potential. arXiv 2021, arXiv:2111.12565. [Google Scholar]
- Matienzo, D.; Bordoni, B. Anatomy, Blood Flow; StatPearls: Treasure Island, San Francisco, CA, USA, 2021. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554457/ (accessed on 6 February 2022).
- Adeniyi, A.G.; Ighalo, J.O. A systematic review of pure metals reinforced plastic composites. Iran. Polym. J. 2021, 30, 751–768. [Google Scholar] [CrossRef]
- Lim, Y.Y. Physical and Mechanical Properties Studies of Low Brass Filler Polymer Reinforced Biomaterial. Ph.D. Thesis, National Defence University of Malaysia, Kuala Lumpur, Malaysia, 2021. [Google Scholar]
- Tarasenko, D.; Meinecke, M. Protein-dependent membrane remodeling in mitochondrial morphology and clathrin-mediated endocytosis. Eur. Biophys. J. 2021, 50, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Park, S.; Mariadoss, A.V.A.; Sathiyaseelan, A.; Veeraraghavan, V.P.; Kim, S.; Wang, M.-H. Chemical composition, antioxidant, and anti-diabetic activities of ethyl acetate fraction of Stachys riederi var. japonica (Miq.) in streptozotocin-induced type 2 diabetic mice. Food Chem. Toxicol. 2021, 155, 112374. [Google Scholar] [CrossRef] [PubMed]
- Abu-Baker, A.N.; Khalil, L.A.; Al-Gonmeen, T. A multi-analytical exploration of the chemical composition, microstructural properties and corrosion inhibiting treatment for an archaeological brass censer from Umm Zuwaytinah, Amman. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms. 2021, 502, 73–79. [Google Scholar] [CrossRef]
- Liu, F.; Hong, T.; Xie, J.; Zhan, X.; Wang, Y. Application of Reactive Oxygen Species-Based Nanomaterials in Dentistry: A Review. Crystals 2021, 11, 266. [Google Scholar] [CrossRef]
- Wu, M.-L.; Tsai, K.-L.; Wang, S.-M.; Wu, J.-C.; Wang, B.-S.; Lee, Y.-T. Mechanism of Hydrogen Peroxide and Hydroxyl Free Radical–Induced Intracellular Acidification in Cultured Rat Cardiac Myoblasts. Circ. Res. 1996, 78, 564–572. [Google Scholar] [CrossRef]
- Christenson, E.M.; Anderson, J.M.; Hiltner, A. Biodegradation mechanisms of polyurethane elastomers. Corros. Eng. Sci. Technol. 2007, 42, 312–323. [Google Scholar] [CrossRef]
- El Awady, M.E.; Asker, M.S.; Haggag, K.M.; Abdelaziz, D.; El-Shall, F.N. Chemical and Biological Assay for The Degradation of Microwave Synthesized-Hyperbranched Poly(Urethane-Urea) by Local Bacterial Isolates. Egypt. J. Chem. 2022, 65, 297–305. [Google Scholar] [CrossRef]
- Stokes, K.; Coury, A.; Urbanski, P. Autooxidative Degradation of Implanted Polyether Polyurethane Devices. J. Biomater. Appl. 1986, 1, 411–448. [Google Scholar] [CrossRef]
- Han, Y.; Lian, M.; Wu, Q.; Qiao, Z.; Sun, B.; Dai, K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 629270. [Google Scholar] [CrossRef]
- DiDomenico, C.D.; Lintz, M.; Bonassar, L.J. Molecular transport in articular cartilage—What have we learned from the past 50 years? Nat. Rev. Rheumatol. 2018, 14, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Jahir-Hussain, M.J.; Maaruf, N.A.; Esa, N.E.F.; Jusoh, N. The effect of pore geometry on the mechanical properties of 3D-printed bone scaffold due to compressive loading. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012016. [Google Scholar] [CrossRef]
- Xie, L.; You, Q.; Wang, E.; Li, T.; Song, Y. Quantitative characterization of pore size and structural features in ultra-low permeability reservoirs based on X-ray computed tomography. J. Pet. Sci. Eng. 2022, 208, 109733. [Google Scholar] [CrossRef]
- Noble, S.; Scheinost, D.; Constable, R.T. A guide to the measurement and interpretation of fMRI test-retest reliability. Curr. Opin. Behav. Sci. 2021, 40, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Charry, E.M.; Baikova, E.; Hilger, A.; Hirn, U.; Schennach, R.; Manke, I.; Schmidt, V.; Zojer, K. Capturing Centimeter-Scale Local Variations in Paper Pore Space via μ-CT: A Benchmark Study Using Calendered Paper. Microsc. Microanal. 2021, 27, 1305–1315. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Sibiryakov, B.; Leite, L.W.B.; Sibiriakov, E. Porosity, specific surface area and permeability in porous media. J. Appl. Geophys. 2021, 186, 104261. [Google Scholar] [CrossRef]
- Miles, C.E.; Lima, M.R.N.; Buevich, F.; Gwin, C.; Murthy, N.S.; Kohn, J. Comprehensive hydrolytic degradation study of a new poly(ester-amide) used for total meniscus replacement. Polym. Degrad. Stab. 2021, 190, 109617. [Google Scholar] [CrossRef]








| Code No. | Magnification | Effective Pore Diameter, nm | Effective Pore Area, nm2 |
|---|---|---|---|
| FDa1 | 20k | 491.3 | 189,545 |
| FDb1 | 20k | 245.6 | 47,386 |
| SDa1 | 20k | 491.3 | 189,545 |
| SDa2 | 20k | 256.8 | 51,792 |
| SDa3 | 20k | 178.6 | 25,064 |
| SDb1 | 20k | 234.5 | 43,176 |
| CDa1 | 10k | 736.9 | 426,477 |
| CDb1 | 10k | 580.6 | 264,737 |
| CDb2 | 10k | 312.6 | 76,758 |
| Calculated Mean | 392.0 | 146,053 | |
| Code No. | Average Pore Area, nm2 | Surface Square Unit Area, nm2 | Porosity, % |
|---|---|---|---|
| CA1 & CA2 | 170,748 | 6,125,000 | 2.79 |
| SA1 & SA2 | 120,669 | 911,250 | 13.24 |
| SA1 & SA3 | 107,305 | 5,120,000 | 2.10 |
| Total Average | 132,907 | 4,052,083 | 6.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.Y.; Miskon, A.; Zaidi, A.M.A.; Megat Ahmad, M.M.H.; Abu Bakar, M. Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications. Materials 2022, 15, 1421. https://doi.org/10.3390/ma15041421
Lim YY, Miskon A, Zaidi AMA, Megat Ahmad MMH, Abu Bakar M. Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications. Materials. 2022; 15(4):1421. https://doi.org/10.3390/ma15041421
Chicago/Turabian StyleLim, Yan Yik, Azizi Miskon, Ahmad Mujahid Ahmad Zaidi, Megat Mohamad Hamdan Megat Ahmad, and Muhamad Abu Bakar. 2022. "Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications" Materials 15, no. 4: 1421. https://doi.org/10.3390/ma15041421
APA StyleLim, Y. Y., Miskon, A., Zaidi, A. M. A., Megat Ahmad, M. M. H., & Abu Bakar, M. (2022). Structural Characterization Analyses of Low Brass Filler Biomaterial for Hard Tissue Implanted Scaffold Applications. Materials, 15(4), 1421. https://doi.org/10.3390/ma15041421