Contribution of Dislocations in SiC Seed Crystals on the Melt-Back Process in SiC Solution Growth
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
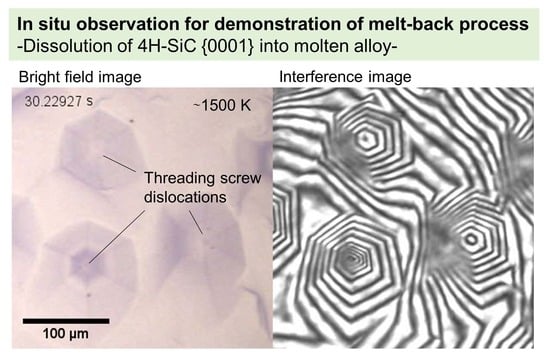
3.1. Dissolution Kinetics of C Face of n-Type 4H-SiC
3.2. Effect of Doping Condition and Polarity of 4H-SiC
4. Conclusions
- (1)
- When n-type SiC with light nitrogen doping was dissolved, hexagonal spiral dissolutions originating from TSD occurred on the C face, and its behavior followed the BCF theory. The dissolution rate decreased as the degree of interface undersaturation decreased, which is accompanied by approaching a smooth interface. On the Si face of the same crystal, the local dissolutions also occurred at TED.
- (2)
- The dissolution behaviors were also clarified for semi-insulating and heavily nitrogen-doped crystals. The occurrence of local dissolutions at the dislocations and the dissolution shape depended significantly on the doping conditions. Since such local dissolution may occur during the melt-back process, it is important to promote the dissolution while maintaining a smooth interface by selecting the seed crystal and controlling the degree of interface undersaturation to the sufficiently low value.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Maan, A.S.; Akhtar, J. Electronic transport in epitaxial 4H–SiC based Schottky diodes modified selectively by swift heavy ions. Mater. Sci. Semicond. Process. 2020, 115, 105108. [Google Scholar] [CrossRef]
- Ramachandran, V.; Brady, M.F.; Smith, A.R.; Feenstra, R.M.; Greve, D.W. Preparation of atomically flat surfaces on silicon carbide using hydrogen etching. J. Electron. Mater. 1998, 27, 308–312. [Google Scholar] [CrossRef]
- Kado, M.; Daikoku, H.; Sakamoto, H.; Suzuki, H.; Bessho, T.; Yashiro, N.; Kusunoki, K.; Okada, N.; Moriguchi, K.; Kamei, K. High-Speed Growth of 4H-SiC Single Crystal Using Si-Cr Based Melt. Mater. Sci. Forum. 2013, 740–742, 73–76. [Google Scholar] [CrossRef]
- Kusunoki, K.; Okada, N.; Kamei, K.; Moriguchi, K.; Daikoku, H.; Kado, M.; Sakamoto, H.; Bessho, T.; Ujihara, T. Top-seeded solution growth of three-inch-diameter 4H-SiC using convection control technique. J. Cryst. Growth 2014, 395, 68–73. [Google Scholar] [CrossRef]
- Daikoku, H.; Kawanishi, S.; Yoshikawa, T. Mechanism of Replicating 4H-SiC Polytype during Solution Growth on Concave Surface. Cryst. Growth Des. 2018, 18, 3820–3826. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Kawanishi, S.; Tanaka, T. Solution growth of silicon carbide using Fe-Si solvent. Jpn. J. Appl. Phys. 2010, 49, 0513021–0513026. [Google Scholar] [CrossRef]
- Kawanishi, S.; Nagamatsu, Y.; Yoshikawa, T.; Shibata, H. Availability of Cr-rich Cr-Si solvent for rapid solution growth of 4H-SiC. J. Cryst. Growth 2020, 549, 125877. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, C.; Harada, S.; Tagawa, M.; Ujihara, T. Application of C-face dislocation conversion to 2 inch SiC crystal growth on an off-axis seed crystal. CrystEngComm 2019, 21, 7260–7265. [Google Scholar] [CrossRef]
- Kusunoki, K.; Seki, K.; Kishida, Y.; Kaido, H.; Moriguchi, K.; Daikoku, H.; Kado, M.; Shirai, T.; Akita, M.; Saito, H. Development of Solvent Inclusion Free 4H-SiC Off-Axis Wafer Grown by the Top-Seeded Solution Growth Technique. Mater. Sci. Forum 2018, 924, 31–34. [Google Scholar] [CrossRef]
- Ariyawong, K.; Shin, Y.J.; Dedulle, J.M.; Chaussende, D. Analysis of Macrostep Formation during Top Seeded Solution Growth of 4H-SiC. Cryst. Growth Des. 2016, 16, 3231–3236. [Google Scholar] [CrossRef]
- Komatsu, N.; Mitani, T.; Hayashi, Y.; Kato, T.; Harada, S.; Ujihara, T.; Okumura, H. Modification of the surface morphology of 4H-SiC by addition of Sn and Al in solution growth with SiCr solvents. J. Cryst. Growth 2017, 458, 37–43. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Seki, K.; Kusunoki, K. Effect of Melt-Back Process on the Quality of Grown Crystal in SiC Solution Growth. Mater. Sci. Forum 2019, 963, 75–79. [Google Scholar] [CrossRef]
- Kawanishi, S.; Yoshikawa, T.; Morita, K.; Kusunoki, K.; Kamei, K.; Suzuki, H.; Sakamoto, H. Real-time observation of the interface between SiC and a liquid alloy and its application to the dissolution behavior of SiC at 1573 K. J. Appl. Phys. 2013, 114, 214313. [Google Scholar] [CrossRef]
- Kawanishi, S.; Kamiko, M.; Yoshikawa, T.; Mitsuda, Y.; Morita, K. Analysis of the Spiral Step Structure and the Initial Solution Growth Behavior of SiC by Real-Time Observation of the Growth Interface. Cryst. Growth Des. 2016, 16, 4822–4830. [Google Scholar] [CrossRef]
- Kawanishi, S.; Yoshikawa, T.; Morita, K. Real-Time Observation of High Temperature Interface between SiC Substrate and Solution during Dissolution of SiC. Mater. Sci. Forum. 2013, 740–742, 35–38. [Google Scholar] [CrossRef]
- Kawanishi, S.; Yoshikawa, T. In Situ Interface Observation of 3C-SiC Nucleation on Basal Planes of 4H-SiC During Solution Growth of SiC from Molten Fe-Si Alloy. JOM 2018, 70, 1239–1247. [Google Scholar] [CrossRef]
- Syväjärvi, M.; Yakimova, R.; Janzén, E. Anisotropic etching of SiC. J. Electrochem. Soc. 2000, 147, 3519–3522. [Google Scholar] [CrossRef]
- Yao, Y.; Ishikawa, Y.; Sato, K.; Sugawara, Y.; Danno, K.; Suzuki, H.; Bessho, T. Dislocation Revelation from (000-1) Carbon-face of 4H-SiC by Using Vaporized KOH at High Temperature. Appllied Phys. Express 2012, 5, 075601. [Google Scholar] [CrossRef]
- Horn, F.H. Screw dislocations, etch figures, and holes. Philos. Mag. 1952, 43, 1210–1213. [Google Scholar] [CrossRef]
- Gilman, J.J.; Johnston, W.G. Observations of dislocation glide and climb in lithium fluoride crystals. J. Appl. Phys. 1956, 27, 1018–1022. [Google Scholar] [CrossRef]
- Ives, M.B.; McAusland, D.D. On the slope of etch pits. Surf. Sci. 1968, 12, 189–207. [Google Scholar] [CrossRef]
- Kawanishi, S.; Yoshikawa, T. Measurement and thermodynamics of the carbon solubilities in molten Si–Fe, Si–Ni, and Si–Cr–Fe alloys at 2073 K. ISIJ Int. 2020, 60, 2123–2128. [Google Scholar] [CrossRef] [Green Version]
- Daikoku, H.; Kawanishi, S.; Yoshikawa, T. Measurement and thermodynamic analysis of carbon solubility in Si–Cr alloys at SiC saturation. Mater. Trans. 2017, 58, 1434–1438. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Ishikawa, Y.; Sugawara, Y.; Saitoh, H.; Danno, K.; Suzuki, H.; Kawai, Y.; Shibata, N. Molten KOH Etching with Na2O2 Additive for Dislocation Revelation in 4H-SiC Epilayers and Substrates. Jpn. J. Appl. Phys. 2011, 50, 075502. [Google Scholar] [CrossRef]
- Daikoku, H.; Kado, M.; Seki, A.; Sato, K.; Bessho, T.; Kusunoki, K.; Kaidou, H.; Kishida, Y.; Moriguchi, K.; Kamei, K. Solution growth on concave surface of 4H-SiC crystal. Cryst. Growth Des. 2016, 16, 1256–1260. [Google Scholar] [CrossRef]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The Growth of Crystals and the Equilibrium Structure of Their Surfaces. Philos. Trans. R. Soc. Lond. Ser. A 1951, 243, 299–358. [Google Scholar]
- Ives, M.B.; Hirth, J.P. Dissolution kinetics at dislocation etch pits in single crystals of lithium fluoride. J. Chem. Phys. 1960, 33, 517–525. [Google Scholar] [CrossRef]
- Cabrera, N.; Levine, M.M. XLV. On the dislocation theory of evaporation of crystals. Philos. Mag. 1956, 1, 450–458. [Google Scholar] [CrossRef]
- Gilman, J.J. Effect of Imperfections on Dissolution. In The Surface Chemistry of Metals and Semiconductors; Gatos, H.C., Ed.; John Wiley & Sons, Inc.: New York, NY, USA; London, UK, 1960; pp. 136–150. [Google Scholar]
- Park, S.H.; Esaka, H.; Shinozuka, K. Equalization of Primary Dendrite Arm Spacing during Growth of Columnar Dendrite. J. Jpn. Inst. Met. 2012, 76, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Wu, P. Etching study of dislocations in heavily nitrogen doped SiC crystals. J. Cryst. Growth 2010, 312, 1193–1198. [Google Scholar] [CrossRef]
- Wu, P.; Yoganathan, M.; Zwieback, I.; Chen, Y.; Dudley, M. Characterization of Dislocations and Micropipes in 4H n + SiC Substrates. Mater. Sci. Forum 2009, 600–603, 333–336. [Google Scholar] [CrossRef]
- Kallinger, B.; Polster, S.; Berwian, P.; Friedrich, J.; Müller, G.; Danilewsky, A.N.; Wehrhahn, A.; Weber, A. Threading dislocations in n- and p-type 4H-SiC material analyzed by etching and synchrotron X-ray topography. J. Cryst. Growth 2011, 314, 21–29. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Bondokov, R.; Soloviev, S.; Sudarshan, T. The Effect of Doping Concentration and Conductivity Type on Preferential Etching of 4H-SiC by Molten KOH. Mater. Res. Soc. Symp. Proc. 2004, 815, J5.20.1–J5.20.5. [Google Scholar] [CrossRef]
- Mitani, T.; Okamura, M.; Takahashi, T. Modulation of Growth Rate by Electric Current in Liquid-Phase Epitaxy of 4H-SiC Direct Current Voltage. Jpn. J. Appl. Phys. 2013, 52, 085503. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawanishi, S.; Shibata, H.; Yoshikawa, T. Contribution of Dislocations in SiC Seed Crystals on the Melt-Back Process in SiC Solution Growth. Materials 2022, 15, 1796. https://doi.org/10.3390/ma15051796
Kawanishi S, Shibata H, Yoshikawa T. Contribution of Dislocations in SiC Seed Crystals on the Melt-Back Process in SiC Solution Growth. Materials. 2022; 15(5):1796. https://doi.org/10.3390/ma15051796
Chicago/Turabian StyleKawanishi, Sakiko, Hiroyuki Shibata, and Takeshi Yoshikawa. 2022. "Contribution of Dislocations in SiC Seed Crystals on the Melt-Back Process in SiC Solution Growth" Materials 15, no. 5: 1796. https://doi.org/10.3390/ma15051796
APA StyleKawanishi, S., Shibata, H., & Yoshikawa, T. (2022). Contribution of Dislocations in SiC Seed Crystals on the Melt-Back Process in SiC Solution Growth. Materials, 15(5), 1796. https://doi.org/10.3390/ma15051796