From Isostructurality to Structural Diversity of Ag(I) Coordination Complexes Formed with Imidazole Based Bipodal Ligands, and Disclosure of a Unique Topology
Abstract
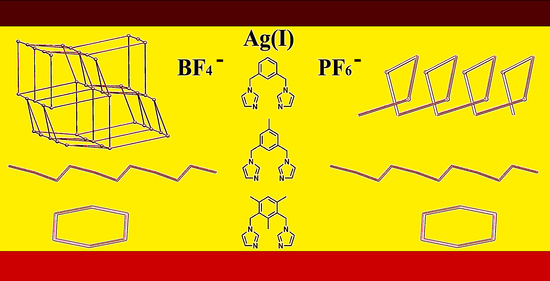
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Measurements
2.3. Synthesis of Ag(I) Complexes
2.4. Structure Determination
3. Results and Discussion
3.1. Crystal Structure of the Ag(I) Complex with BF4− as Counterion ({[Ag2(bib)3](BF4)2}n, 1)
3.2. Crystal Structure of the Ag(I) Complex with PF6− as Counterion ({[Ag(bib)]PF6}n, 2)
3.3. Effect of Modifying the Ligand on the Resulting Crystal Structure
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowskill, D.H.; Sugden, I.J.; Konstantinopoulos, S.; Adjiman, C.S.; Pantelides, C.C. Crystal structure prediction methods for organic molecules: State of the art. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Price, S.L.; Brandenburg, J.G. Non-Covalent Interactions in Quantum Chemistry and Physics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 333–363. [Google Scholar]
- Bombicz, P. The way from isostructurality to polymorphism. Where are the borders? The role of supramolecular interactions and crystal symmetries. Crystallogr. Rev. 2017, 23, 118–151. [Google Scholar] [CrossRef]
- Kálmán, A.; Párkányi, L.; Argay, G. Classification of the Isostructurality of Organic Molecules in the Crystalline State. Acta Crystallogr. 1993, B49, 1039–1049. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef]
- Arhangelskis, M.; Van Meervelt, L.; Dobrzańska, L. Influence of ligand composition on crystal structure formation–isostructurality and morphotropism. CrystEngComm 2021, 23, 317–323. [Google Scholar] [CrossRef]
- Dobrzańska, L. Counterion effect and isostructurality in a series of Ag(I) complexes containing a flexible, imidazole based dipodal ligand. Materials 2021, 14, 1804. [Google Scholar] [CrossRef]
- Dobrzańska, L. Anion directed supramolecular architectures of silver(I) complexes with 1,3-bis(imidazol-1-ylmethyl)-2,4,6- trimethylbenzene and a reversible, solvent-induced structural change during a single-crystal-to-single-crystal transformation. CrystEngComm 2011, 13, 2303–2309. [Google Scholar] [CrossRef]
- Alen, J.; Van Meervelt, L.; Dehaen, W.; Dobrzańska, L. Solvent diffusion through a non-porous crystal ‘caught in the act’ and related single-crystal-to-single-crystal transformations in a cationic dinuclear Ag(I) complex. CrystEngComm 2015, 17, 8957–8964. [Google Scholar] [CrossRef]
- Dobrzańska, L. Concomitant, genuine 1D supramolecular isomers of an Ag(I) complex with 1,3-bis(imidazol-1-ylmethyl)-2,4,6-trimethylbenzene and BF4− as counterion. Inorg. Chem. Commun. 2015, 55, 21–24. [Google Scholar] [CrossRef]
- APEX2, Bruker Analytical X-ray; Instruments Inc.: Madison, WI, USA, 2009.
- SAINT, Bruker Analytical X-ray; Instruments Inc.: Madison, WI, USA, 2009.
- SADABS, Bruker Analytical X-ray; Instruments Inc.: Madison, WI, USA, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Persistence of Vision. Available online: www.povray.org (accessed on 28 December 2021).
- Lin, J.-D.; Li, Z.-H.; Li, J.-R.; Du, S.-W. Synthesis and crystal structures of three novel coordination polymers generated from AgCN and AgSCN with flexible N-donor ligands. Polyhedron 2007, 26, 107–114. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Shevchenko, A.P.; Blatov, V.A. Topological databases: Why do we need them for design of coordination polymers? Cryst. Growth Des. 2019, 19, 2604–2614. [Google Scholar] [CrossRef]
- Takeda, K.; Fujisawa, M.; Makino, T.; Yoshii, E.; Yamaguchi, K. Reaction of β-heteroatom-substituted α, β-unsaturated acylsilanes with ketone enolates: A new [3+2] annulation based on the Brook rearrangement. J. Am. Chem. Soc. 1993, 115, 9351–9352. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Islam, N.; Hashizume, D.; Izumi, F.; Fujita, M.; Kawano, M. Dramatic structural rearrangements in porous coordination networks. J. Am. Chem. Soc. 2011, 133, 5853–5860. [Google Scholar] [CrossRef]
- Ohmori, O.; Kawano, M.; Fujita, M. Crystal-to-crystal guest exchange of large organic molecules within a 3D coordination network. J. Am. Chem. Soc. 2004, 126, 16292–16293. [Google Scholar] [CrossRef]
- Hoshino, M.; Khutia, A.; Xing, H.; Inokuma, Y.; Fujita, M. The crystalline sponge method updated. IUCrJ 2016, 3, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2006; p. 13. [Google Scholar]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer, version 3.1; University of Western Australia: Crawley, WA, Australia, 2012. [Google Scholar]








| Compound Reference | 1 | 2 |
|---|---|---|
| Chemical formula | C42H42Ag2B2F8N12 | C14H14AgF6N4P |
| Formula mass | 1104.23 | 491.13 |
| Crystal system | Monoclinic | Orthorombic |
| a/Å | 6.9785(8) | 13.3957(19) |
| b/Å | 32.556(4) | 10.5887(15) |
| c/Å | 19.241(2) | 11.8827(17) |
| α/° | 90 | 90 |
| β/° | 93.561(2) | 90 |
| γ/° | 90 | 90 |
| Unit cell volume/Å3 | 4363.0(9) | 1685.5(4) |
| Temperature/K | 100(2) | 100(2) |
| Space group | Cc | Pnna |
| No. of formula units per unit cell, Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| Absorption coefficient, μ/mm−1 | 0.980 | 1.359 |
| No. of reflections measured | 13683 | 9153 |
| No. of independent reflections | 8270 | 1765 |
| Rint | 0.0320 | 0.0438 |
| Final R1 a values (I > 2σ(I)) | 0.0478 | 0.0562 |
| Final wR2 b values (I > 2σ(I)) | 0.1112 | 0.1288 |
| Final R1 a values (all data) | 0.0513 | 0.0747 |
| Final wR2 b values (all data) | 0.1136 | 0.1395 |
| Goodness of fit on F2 | 1.049 | 1.043 |
| D-H⋯A | H⋯A/Å | D···A/Å | D-H···A/° |
|---|---|---|---|
| C5-H5⋯F12 i | 2.54 | 3.149 (7) | 122 |
| C6-H6A⋯F12 ii | 2.47 | 3.434 (7) | 164 |
| C8-H8⋯F13 iii | 2.36 | 3.280 (1) | 162 |
| C2-H2⋯F14 | 2.61 | 3.405 (16) | 141 |
| C4-H4⋯F15 iv | 2.68 | 3.444 (11) | 137 |
| C2-H2⋯F16 v | 2.31 | 3.150 (2) | 147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzańska, L. From Isostructurality to Structural Diversity of Ag(I) Coordination Complexes Formed with Imidazole Based Bipodal Ligands, and Disclosure of a Unique Topology. Materials 2022, 15, 1852. https://doi.org/10.3390/ma15051852
Dobrzańska L. From Isostructurality to Structural Diversity of Ag(I) Coordination Complexes Formed with Imidazole Based Bipodal Ligands, and Disclosure of a Unique Topology. Materials. 2022; 15(5):1852. https://doi.org/10.3390/ma15051852
Chicago/Turabian StyleDobrzańska, Liliana. 2022. "From Isostructurality to Structural Diversity of Ag(I) Coordination Complexes Formed with Imidazole Based Bipodal Ligands, and Disclosure of a Unique Topology" Materials 15, no. 5: 1852. https://doi.org/10.3390/ma15051852
APA StyleDobrzańska, L. (2022). From Isostructurality to Structural Diversity of Ag(I) Coordination Complexes Formed with Imidazole Based Bipodal Ligands, and Disclosure of a Unique Topology. Materials, 15(5), 1852. https://doi.org/10.3390/ma15051852