Decellularized Pig Kidney with a Micro-Nano Secondary Structure Contributes to Tumor Progression in 3D Tumor Model
Abstract
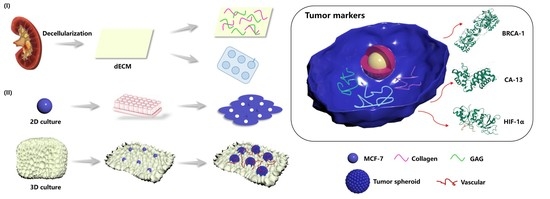
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Acellular Porcine Kidney Matrix Scaffold
2.3. Analysis of the Micro-Nano Structure of the Porcine Kidney-Derived Decellularized Matrix Scaffold
2.4. Decellularization Efficiency Test
2.5. Physical Performance Test of Pig Kidney Matrix Scaffold
2.5.1. Water Absorption Rate
2.5.2. Porosity
2.5.3. Contact Angle Detection
2.5.4. Infrared Spectrum Detection
2.5.5. Compressive Modulus Test
2.5.6. Thermal Stability Analysis
2.6. Biocompatibility
2.7. Construction of Breast Cancer Tumor Model
2.7.1. Growth Status of Breast Cancer Cells (MCF-7) on the Kidney Matrix Scaffold
2.7.2. Permeability of Breast Cancer Cells (MCF-7) on the Porcine Kidney Matrix Scaffold
2.7.3. The Size of Tumor Spheres in Breast Cancer
2.7.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Decellularization Efficiency of the Porcine Kidney Matrix
3.2. Microstructure and Elemental Analysis of the Pig Kidney-Derived Matrix Scaffold
3.3. Physical Properties of the Pig Kidney Matrix Scaffolds
3.3.1. Porosity of the Kidney-Derived Matrix
3.3.2. Water Absorption and Contact Angle of the Pig Kidney-Derived Matrix Scaffolds
3.3.3. Compression Modulus of the Pig Kidney-Derived Matrix Scaffold
3.3.4. Thermal Stability of the Pig Kidney-Derived Matrix Scaffolds
3.3.5. Infrared Spectra of Pig Kidney-Derived Matrix Scaffolds
3.4. Biocompatibility of Pig Kidney Matrix Scaffolds
3.5. Construction of the Breast Cancer Tumor Model
3.5.1. Growth of MCF-7 Cells in 2D and 3D Environments
3.5.2. Infiltration of MCF-7 Cells in Pig Renal Scaffolds
3.5.3. Investigation of Tumor Sphere Size
3.5.4. HIF-1α and BRCA1 Expression in 2D and 3D Environments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Phan-Lai, V.; Florczyk, S.J.; Kievit, F.M.; Wang, K.; Gad, E.; Disis, M.L.; Zhang, M.; Disis, N.L. Three-Dimensional Scaffolds to Evaluate Tumor Associated Fibroblast-Mediated Suppression of Breast Tumor Specific T Cells. Biomacromolecules 2013, 14, 1330–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite Hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Liao, W.-C.; Sohn, Y.S.; Fadeev, M.; Cecconello, A.; Nechushtai, R.; Willner, I. Stimuli-Responsive Nucleic Acid-Based Polyacrylamide Hydrogel-Coated Metal-Organic Framework Nanoparticles for Controlled Drug Release. Adv. Funct. Mater. 2018, 28, 1705137. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Yang, S.; Wang, S.; Zhang, C.; Wang, H.; Cheng, Y.Y.; Wang, Y.; Liu, T.; Song, K. A novel tissue-engineered 3D tumor model for anti-cancer drug discovery. Biofabrication 2019, 11, 015004. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Halper, J. Basic Components of Connective Tissues and Extracellular Matrix: Fibronectin, Fibrinogen, Laminin, Elastin, Fibrillins, Fibulins, Matrilins, Tenascins and Thrombospondins. In Progress in Heritable Soft Connective Tissue Diseases; Springer: Cham, Switzerland, 2021; Volume 1348, pp. 105–126. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [Green Version]
- Ross, E.A.; Williams, M.J.; Hamazaki, T.; Terada, N.; Clapp, W.L.; Adin, C.; Ellison, G.W.; Jorgensen, M.; Batich, C.D. Embryonic Stem Cells Proliferate and Differentiate when Seeded into Kidney Scaffolds. J. Am. Soc. Nephrol. 2009, 20, 2338–2347. [Google Scholar] [CrossRef] [Green Version]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [Green Version]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [Green Version]
- Lou, J.; Stowers, R.; Nam, S.; Xia, Y.; Chaudhuri, O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 2018, 154, 213–222. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, S.; Liu, Y.; Sun, C.; Cheng, G.; Luan, Y.; Li, K.; Wang, J.; Xie, X.; Zhao, S. Porcine kidneys as a source of ECM scaffold for kidney regeneration. Mater. Sci. Eng. C 2015, 56, 451–456. [Google Scholar] [CrossRef]
- Remuzzi, A.; Figliuzzi, M.; Bonandrini, B.; Silvani, S.; Azzollini, N.; Nossa, R.; Benigni, A.; Remuzzi, G. Experimental Evaluation of Kidney Regeneration by Organ Scaffold Recellularization. Sci. Rep. 2017, 7, srep43502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodička, P.; Smetana, J.K.; Dvořánková, B.; Emerick, T.; Xu, Y.Z.; Ourednik, J.; Ourednik, V.; Motlík, J. The Miniature Pig as an Animal Model in Biomedical Research. Ann. N. Y. Acad. Sci. 2005, 1049, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Pantel, K.; Kang, Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 2013, 19, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Wang, S.; Xing, Y.; Wang, H.; Nie, Y.; Liu, T.; Song, K. Multiple comparisons of three different sources of biomaterials in the application of tumor tissue engineering in vitro and in vivo. Int. J. Biol. Macromol. 2019, 130, 166–176. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Li, Y.; Song, K. Cytotoxicity and growth-inhibiting activity of Astragalus polysaccharides against breast cancer via the regulation of EGFR and ANXA1. J. Nat. Med. 2021, 75, 854–870. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, S.; Liu, H.; Wang, Y.; Jiao, Z.; Nie, Y.; Wang, H.; Liu, T.; Song, K. Evaluation of anti-tumor effects of crocin on a novel 3D tissue-engineered tumor model based on sodium alginate/gelatin microbead. Int. J. Biol. Macromol. 2021, 174, 339–351. [Google Scholar] [CrossRef]
- Sobral, J.M.; Caridade, S.; Sousa, R.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Pr, A.K.; Yoo, J.J.; Zahran, F.; Atala, A.; Lee, S.J. A Photo-Crosslinkable Kidney ECM-Derived Bioink Accelerates Renal Tissue Formation. Adv. Healthc. Mater. 2019, 8, 1800992. [Google Scholar] [CrossRef]
- Hoshiba, T. Decellularized Extracellular Matrix for Cancer Research. Materials 2019, 12, 1311. [Google Scholar] [CrossRef] [Green Version]
- Poornejad, N.; Nielsen, J.; Morris, R.J.; Gassman, J.R.; Reynolds, P.; Roeder, B.L.; Cook, A.D. Comparison of four decontamination treatments on porcine renal decellularized extracellular matrix structure, composition, and support of renal tubular epithelium cells. J. Biomater. Appl. 2016, 30, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Junka, R.; Yu, X. Polymeric nanofibrous scaffolds laden with cell-derived extracellular matrix for bone regeneration. Mater. Sci. Eng. C 2020, 113, 110981. [Google Scholar] [CrossRef]
- Liu, L.; Yoshioka, M.; Nakajima, M.; Ogasawara, A.; Liu, J.; Hasegawa, K.; Li, S.; Zou, J.; Nakatsuji, N.; Kamei, K.-I.; et al. Nanofibrous gelatin substrates for long-term expansion of human pluripotent stem cells. Biomaterials 2014, 35, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Saleh, T.; Ahmed, E.; Kwak, H.-H.; Park, K.-M.; Yang, S.-R.; Kang, B.-J.; Choi, K.-Y.; Kang, K.-S.; Woo, H.-M. Biocompatibility and hemocompatibility of efficiently decellularized whole porcine kidney for tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 2034–2047. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Kuroda, K.; Otaka, Y.; Kinoshita, R.; Kita, M.; Oyamada, T.; Kanai, K. Decellularization of canine kidney for three-dimensional organ regeneration. Vet. World 2020, 13, 452–457. [Google Scholar] [CrossRef]
- Berrier, A.L.; Yamada, K.M. Cell-matrix adhesion. J. Cell. Physiol. 2007, 213, 565–573. [Google Scholar] [CrossRef]
- Lv, K.; Zhu, J.; Zheng, S.; Jiao, Z.; Nie, Y.; Song, F.; Liu, T.; Song, K. Evaluation of inhibitory effects of geniposide on a tumor model of human breast cancer based on 3D printed Cs/Gel hybrid scaffold. Mater. Sci. Eng. C 2021, 119, 111509. [Google Scholar] [CrossRef]
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. 2021, 187, 840–849. [Google Scholar] [CrossRef]
- Lih, E.; Park, K.W.; Chun, S.Y.; Kim, H.; Kwon, T.G.; Joung, Y.K.; Han, D.K. Biomimetic Porous PLGA Scaffolds Incorporating Decellularized Extracellular Matrix for Kidney Tissue Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 21145–21154. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananthanarayanan, B.; Kim, Y.; Kumar, S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials 2011, 32, 7913–7923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poornejad, N.; Frost, T.S.; Scott, D.R.; Elton, B.B.; Reynolds, P.R.; Roeder, B.L.; Cook, A.D. Freezing/Thawing without Cryoprotectant Damages Native but not Decellularized Porcine Renal Tissue. Organogenesis 2015, 11, 30–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vásquez-Rivera, A.; Oldenhof, H.; Hilfiker, A.; Wolkers, W.F. Spectral fingerprinting of decellularized heart valve scaffolds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 95–102. [Google Scholar] [CrossRef]
- Zhao, F.; Cheng, J.; Sun, M.; Yu, H.; Wu, N.; Li, Z.; Zhang, J.; Li, Q.; Yang, P.; Liu, Q.; et al. Digestion degree is a key factor to regulate the printability of pure tendon decellularized extracellular matrix bio-ink in extrusion-based 3D cell printing. Biofabrication 2020, 12, 045011. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Zhu, Y.-X.; Ma, H.-C.; Chen, S.-N.; Chao, J.-Y.; Ruan, W.-D.; Wang, D.; Du, F.-G.; Meng, Y.-Z. Developing multi-cellular tumor spheroid model (MCTS) in the chitosan/collagen/alginate (CCA) fibrous scaffold for anticancer drug screening. Mater. Sci. Eng. C 2016, 62, 215–225. [Google Scholar] [CrossRef]
- Chaji, S.; Al-Saleh, J.; Gomillion, C. Bioprinted Three-Dimensional Cell-Laden Hydrogels to Evaluate Adipocyte-Breast Cancer Cell Interactions. Gels 2020, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Qin, S.; Peng, J.; Chen, A.; Nie, Y.; Liu, T.; Song, K. Engineering gelatin-based alginate/carbon nanotubes blend bioink for direct 3D printing of vessel constructs. Int. J. Biol. Macromol. 2020, 145, 262–271. [Google Scholar] [CrossRef]
- Baker, B.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Fong, E.L.; Harrington, D.A.; Farach-Carson, M.C.; Yu, H. Heralding a new paradigm in 3D tumor modeling. Biomaterials 2016, 108, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Feder-Mengus, C.; Ghosh, S.; Reschner, A.; Martin, I.; Spagnoli, G.C. New dimensions in tumor immunology: What does 3D culture reveal? Trends Mol. Med. 2008, 14, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Del Bufalo, F.; Manzo, T.; Hoyos, V.; Yagyu, S.; Caruana, I.; Jacot, J.; Benavides, O.; Rosen, D.; Brenner, M.K. 3D modeling of human cancer: A PEG-fibrin hydrogel system to study the role of tumor microenvironment and recapitulate the in vivo effect of oncolytic adenovirus. Biomaterials 2016, 84, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Gaspar, V.; Mano, J. Bioinstructive microparticles for self-assembly of mesenchymal stem Cell-3D tumor spheroids. Biomaterials 2018, 185, 155–173. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Zhuang, T.; Lu, Y.; Liu, T.; Zhao, W.; Lin, B.; Luo, Y.; Zhang, X. 3D bioprinted breast tumor model for structure–activity relationship study. Bio-Design Manuf. 2020, 3, 361–372. [Google Scholar] [CrossRef]
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.-Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D Bioprinted Vascularized Tumour for Drug Testing. Int. J. Mol. Sci. 2020, 21, 2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szot, C.S.; Buchanan, C.F.; Freeman, J.W.; Rylander, M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011, 32, 7905–7912. [Google Scholar] [CrossRef] [Green Version]
- Lanz, H.L.; Saleh, A.; Kramer, B.; Cairns, J.; Ng, C.P.; Yu, J.; Trietsch, S.J.; Hankemeier, T.; Joore, J.; Vulto, P.; et al. Therapy response testing of breast cancer in a 3D high-throughput perfused microfluidic platform. BMC Cancer 2017, 17, 709. [Google Scholar] [CrossRef] [Green Version]
- Tamura, R.; Tanaka, T.; Akasaki, Y.; Murayama, Y.; Yoshida, K.; Sasaki, H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med. Oncol. 2020, 37, 2. [Google Scholar] [CrossRef] [Green Version]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Wang, H.; Parungao, R.; Wang, Y.; Liu, T.; Song, K. Detection and Evaluation of Anti-Cancer Efficiency of Astragalus Polysaccharide Via a Tissue Engineered Tumor Model. Macromol. Biosci. 2018, 18, e1800223. [Google Scholar] [CrossRef]








| Vibration Peak (cm−1) | Vibrational Bands |
|---|---|
| 3454 | N–H stretching vibration (hydrogen bond) of amide A |
| 2922 | C–H stretching vibration of amide II band |
| 1661 | C=O stretching vibration, COO- with α helix antisymmetric contraction vibration |
| 1558 | N–H stretching vibration |
| 1456 | CH2- or -CH3- bending vibration |
| 1240 | N–H stretching vibration of amide III band |
| 1080 | C–N stretching vibration or N–H stretching vibration peak of the amide IV band |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zheng, L.; Chen, Z.; Jiao, Z.; Liu, T.; Nie, Y.; Kang, Y.; Pan, B.; Song, K. Decellularized Pig Kidney with a Micro-Nano Secondary Structure Contributes to Tumor Progression in 3D Tumor Model. Materials 2022, 15, 1935. https://doi.org/10.3390/ma15051935
Yang S, Zheng L, Chen Z, Jiao Z, Liu T, Nie Y, Kang Y, Pan B, Song K. Decellularized Pig Kidney with a Micro-Nano Secondary Structure Contributes to Tumor Progression in 3D Tumor Model. Materials. 2022; 15(5):1935. https://doi.org/10.3390/ma15051935
Chicago/Turabian StyleYang, Shuangjia, Le Zheng, Zilong Chen, Zeren Jiao, Tianqing Liu, Yi Nie, Yue Kang, Bo Pan, and Kedong Song. 2022. "Decellularized Pig Kidney with a Micro-Nano Secondary Structure Contributes to Tumor Progression in 3D Tumor Model" Materials 15, no. 5: 1935. https://doi.org/10.3390/ma15051935
APA StyleYang, S., Zheng, L., Chen, Z., Jiao, Z., Liu, T., Nie, Y., Kang, Y., Pan, B., & Song, K. (2022). Decellularized Pig Kidney with a Micro-Nano Secondary Structure Contributes to Tumor Progression in 3D Tumor Model. Materials, 15(5), 1935. https://doi.org/10.3390/ma15051935