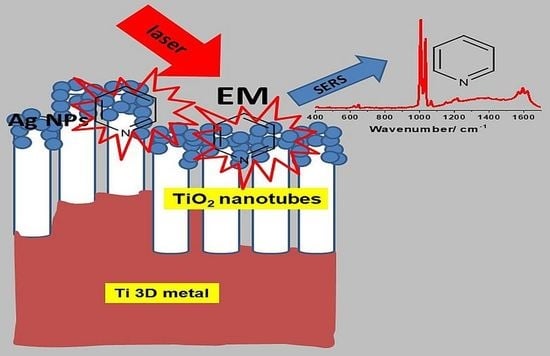
Nanofunctionalization of Additively Manufactured Titanium Substrates for Surface-Enhanced Raman Spectroscopy Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of 3D Titanium Substrates by Powder Bed Fusion Process Using a Laser Beam
2.2. Formation of TiO2 Nanotubes on Ti Substrates Produced by PBF-LB Process
2.3. Deposition of Metal Nanoparticles
2.4. Characterization
3. Results and Discussion
- -
- “With first-generation hot spots that appear as a result of interaction between a single plasmonic nano-object and incident radiation;
- -
- With second-generation hot spots that are produced by coupled plasmonic nano-objects with controllable interparticle distances;
- -
- With third-generation hot spots that are a product of superposition of electromagnetic field originating from metal NPs and electromagnetic field scattered from the backing platform [9]”.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pettinger, B.; Wetzel, H. Surface Enhanced Raman Scattering from Pyridine, Water, and Halide Ions on Au, Ag, and Cu Electrodes. Berichte der Bunsengesellschaft für Phys. Chemie 1981, 85, 473–481. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, R.; Wang, Q.; Xiang, Z.; Li, Z.; Gu, H.; Wang, X. An Overview of Surface-Enhanced Raman Scattering Substrates by Pulsed Laser Deposition Technique: Fundamentals and Applications. Adv. Compos. Hybrid Mater. 2021, 4, 885–905. [Google Scholar] [CrossRef]
- Mosier-Boss, P. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, Applications, and the Future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-Enhanced Raman Spectroscopy: A Brief Perspective. In Surface-Enhanced Raman Scattering; Springer: Berlin/Heidelberg, Germany, 1977; pp. 1–17. [Google Scholar]
- Shvalya, V.; Filipič, G.; Zavašnik, J.; Abdulhalim, I.; Cvelbar, U. Surface-Enhanced Raman Spectroscopy for Chemical and Biological Sensing Using Nanoplasmonics: The Relevance of Interparticle Spacing and Surface Morphology. Appl. Phys. Rev. 2020, 7, 031307. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Schlücker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temperini, M.L.A.; Sala, D.; Lacconi, G.I.; Gioda, A.S.; Macagno, V.A.; Arvia, A.J. Correlation between SERS of Pyridine and Electrochemical Response of Silver Electrodes in Halide-Free Alkaline Solutions. Langmuir 1988, 4, 1032–1039. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; ISBN 9780471607311. [Google Scholar]
- Dong, Y.; Ji, X.; Laaksonen, A.; Cao, W.; An, R.; Lu, L.; Lu, X. Determination of the Small Amount of Proteins Interacting with TiO2 Nanotubes by AFM-Measurement. Biomaterials 2019, 192, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Domańska, M. An Overview of Anodic Oxides Derived Advanced Nanocomposites Substrate for Surface Enhance Raman Spectroscopy. In Assorted Dimensional Reconfigurable Materials; IntechOpen: London, UK, 2020. [Google Scholar]
- Ling, Y.; Zhuo, Y.; Huang, L.; Mao, D. Using Ag-Embedded TiO2 Nanotubes Array as Recyclable SERS Substrate. Appl. Surf. Sci. 2016, 388, 169–173. [Google Scholar] [CrossRef]
- Jimenez-Cisneros, J.; Galindo-Lazo, J.P.; Mendez-Rojas, M.A.; Campos-Delgado, J.R.; Cerro-Lopez, M. Plasmonic Spherical Nanoparticles Coupled with Titania Nanotube Arrays Prepared by Anodization as Substrates for Surface-Enhanced Raman Spectroscopy Applications: A Review. Molecules 2021, 26, 7443. [Google Scholar] [CrossRef]
- Lamberti, A.; Virga, A.; Chiadò, A.; Chiodoni, A.; Bejtka, K.; Rivolo, P.; Giorgis, F. Ultrasensitive Ag-Coated TiO2 Nanotube Arrays for Flexible SERS-Based Optofluidic Devices. J. Mater. Chem. C 2015, 3, 6868–6875. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Lewandowska, M.; Dolata, M.; Janik-Czachor, M. Raman Investigations of TiO2 Nanotube Substrates Covered with Thin Ag or Cu Deposits. J. Raman Spectrosc. 2009, 40, 1652–1656. [Google Scholar] [CrossRef]
- Jaitpal, S.; Chavva, S.R.; Mabbott, S. 3D Printed SERS-Active Thin-Film Substrates Used to Quantify Levels of the Genotoxic Isothiazolinone. ACS Omega 2022, 7, 2850–2860. [Google Scholar] [CrossRef]
- Mersagh Dezfuli, S.; Sabzi, M. Deposition of Ceramic Nanocomposite Coatings by Electroplating Process: A Review of Layer-Deposition Mechanisms and Effective Parameters on the Formation of the Coating. Ceram. Int. 2019, 45, 21835–21842. [Google Scholar] [CrossRef]
- Gross, B.; Lockwood, S.Y.; Spence, D.M. Recent Advances in Analytical Chemistry by 3D Printing. Anal. Chem. 2017, 89, 57–70. [Google Scholar] [CrossRef]
- Lee, S.; Ongko, A.; Kim, H.Y.; Yim, S.-G.; Jeon, G.; Jeong, H.J.; Lee, S.; Kwak, M.; Yang, S.Y. Sub-100 Nm Gold Nanohole-Enhanced Raman Scattering on Flexible PDMS Sheets. Nanotechnology 2016, 27, 315301. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Sigle, D.O.; Herrmann, L.O.; Wolverson, D.; Baumberg, J.J. Nanoimprint Lithography of Al Nanovoids for Deep-UV SERS. ACS Appl. Mater. Interfaces 2014, 6, 17358–17363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chemie Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. [Google Scholar] [CrossRef]
- Pisarek, M.; Holdynski, M.; Roguska, A.; Kudelski, A.; Janik-Czachor, M. TiO2 and Al2O3 Nanoporous Oxide Layers Decorated with Silver Nanoparticles—Active Substrates for SERS Measurements. J. Solid State Electrochem. 2014, 18, 3099–3109. [Google Scholar] [CrossRef] [Green Version]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 Nanotubes: Self-Organized Electrochemical Formation, Properties and Applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Scaramuzzo, F.A.; Dell’Era, A.; Tarquini, G.; Caminiti, R.; Ballirano, P.; Pasquali, M. Phase Transition of TiO2 Nanotubes: An X-ray Study as a Function of Temperature. J. Phys. Chem. C 2017, 121, 24871–24876. [Google Scholar] [CrossRef]
- Chmielewska, A.; Wysocki, B.; Żrodowski, Ł.; Święszkowski, W. Hybrid Solid-Porous Titanium Scaffolds. Trans. Addit. Manuf. Meets Med. 2019, 1, 2–3. [Google Scholar] [CrossRef]
- Wysocki, B.; Idaszek, J.; Zdunek, J.; Rożniatowski, K.; Pisarek, M.; Yamamoto, A.; Święszkowski, W. The Influence of Selective Laser Melting (SLM) Process Parameters on In-Vitro Cell Response. Int. J. Mol. Sci. 2018, 19, 1619. [Google Scholar] [CrossRef] [Green Version]
- Lavery, N.P.; Brown, S.G.R.; Sienz, J.; Cherry, J. A Review of Computational Modelling of Additive Layer Manufacturing—Multi-Scale and Multi-Physics. Sustain. Des. Manuf. 2014, 1, 651–673. [Google Scholar] [CrossRef]
- Li, Y.; Gu, D. Parametric Analysis of Thermal Behavior during Selective Laser Melting Additive Manufacturing of Aluminum Alloy Powder. Mater. Des. 2014, 63, 856–867. [Google Scholar] [CrossRef]
- Chmielewska, A.; Jahadakbar, A.; Wysocki, B.; Elahinia, M.; Święszkowski, W.; Dean, D. Chemical Polishing of Additively Manufactured, Porous, Nickel–Titanium Skeletal Fixation Plates. 3D Print. Addit. Manuf. 2021. [Google Scholar] [CrossRef]
- Nasab, M.H.; Gastaldi, D.; Lecis, N.F.; Vedani, M. On Morphological Surface Features of the Parts Printed by Selective Laser Melting (SLM). Addit. Manuf. 2018, 24, 373–377. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Wang, D.; Han, C.; Li, J.; Wu, C.; Xu, K. Fabrication of Highly Uniform Ag Nanoparticle-TiO2 Nanosheets Array Hybrid as Reusable SERS Substrates. Colloid Interface Sci. Commun. 2020, 39, 100324. [Google Scholar] [CrossRef]
- Krajczewski, J.; Ambroziak, R.; Kudelski, A. Substrates for Surface-Enhanced Raman Scattering Formed on Nanostructured Non-Metallic Materials: Preparation and Characterization. Nanomaterials 2020, 11, 75. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Belcarz, A.; Marcon, L.; Holdynski, M.; Andrzejczuk, M.; Janik-Czachor, M. Improvement of the Bio-Functional Properties of TiO2 Nanotubes. Appl. Surf. Sci. 2016, 388, 775–785. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Pisarek, M.; Roguska, A.; Kudelski, A.; Andrzejczuk, M.; Janik-Czachor, M.; Kurzydłowski, K.J. The Role of Ag Particles Deposited on TiO2 or Al2O3 Self-Organized Nanoporous Layers in Their Behavior as SERS-Active and Biomedical Substrates. Mater. Chem. Phys. 2013, 139, 55–65. [Google Scholar] [CrossRef]
- Roguska, A.; Pisarek, M.; Andrzejczuk, M.; Dolata, M.; Lewandowska, M.; Janik-Czachor, M. Characterization of a Calcium Phosphate–TiO2 Nanotube Composite Layer for Biomedical Applications. Mater. Sci. Eng. C 2011, 31, 906–914. [Google Scholar] [CrossRef]
- Roguska, A.; Kudelski, A.; Pisarek, M.; Opara, M.; Janik-Czachor, M. Surface-Enhanced Raman Scattering (SERS) Activity of Ag, Au and Cu Nanoclusters on TiO2-Nanotubes/Ti Substrate. Appl. Surf. Sci. 2011, 257, 8182–8189. [Google Scholar] [CrossRef]
- Kudelski, A. Raman Spectroscopy of Surfaces. Surf. Sci. 2009, 603, 1328–1334. [Google Scholar] [CrossRef]
- Samriti; Rajput, V.; Gupta, R.K.; Prakash, J. Engineering Metal Oxide Semiconductor Nanostructures for Enhanced Charge Transfer: Fundamentals and Emerging SERS Applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar] [CrossRef]
- Hajipour, P.; Bahrami, A.; Mehr, M.Y.; van Driel, W.D.; Zhang, K. Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B. Materials 2021, 14, 763. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Eden Praire, MN, USA, 1992. [Google Scholar]
- Jiang, X.; Wang, Y.; Pan, C. High Concentration Substitutional N-Doped TiO2 Film: Preparation, Characterization, and Photocatalytic Property. J. Am. Ceram. Soc. 2011, 94, 4078–4083. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C.; Rajendran, N. Effect of Plasma Nitriding on Structure and Biocompatibility of Self-Organised TiO2 Nanotubes on Ti–6Al–7Nb. RSC Adv. 2015, 5, 41763–41771. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Sait, R.; Govindarajan, S.; Cross, R. Nitridation of Optimised TiO2 Nanorods through PECVD towards Neural Electrode Application. Materialia 2018, 4, 127–138. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and Mechanical Properties Investigation of CP Titanium Processed by Selective Laser Melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Stevie, F.A.; Donley, C.L. Introduction to X-ray Photoelectron Spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 063204. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray Photoelectron Spectroscopy: Towards Reliable Binding Energy Referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J. An X-ray Photoelectron Spectroscopy Sputter Profile Study of the Native Air-Formed Oxide Film on Titanium. Appl. Surf. Sci. 1999, 143, 92–100. [Google Scholar] [CrossRef]
- Pisarek, M.; Krawczyk, M.; Hołdyński, M.; Lisowski, W. Plasma Nitriding of TiO2 Nanotubes: N-Doping in Situ Investigations Using XPS. ACS Omega 2020, 5, 8647–8658. [Google Scholar] [CrossRef] [Green Version]
- Ambroziak, R.; Hołdyński, M.; Płociński, T.; Pisarek, M.; Kudelski, A. Cubic Silver Nanoparticles Fixed on TiO2 Nanotubes as Simple and Efficient Substrates for Surface Enhanced Raman Scattering. Materials 2019, 12, 3373. [Google Scholar] [CrossRef] [Green Version]
- Zuo, C.; Jagodzinski, P.W. Surface-Enhanced Raman Scattering of Pyridine Using Different Metals: Differences and Explanation Based on the Selective Formation of α-Pyridyl on Metal Surfaces. J. Phys. Chem. B 2005, 109, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, A.; Pettinger, B. SERS on Carbon Chain Segments: Monitoring Locally Surface Chemistry. Chem. Phys. Lett. 2000, 321, 356–362. [Google Scholar] [CrossRef]
- Pisarek, M.; Krajczewski, J.; Hołdyński, M.; Płociński, T.; Krawczyk, M.; Kudelski, A.; Janik-Czachor, M. Titanium (IV) Oxide Nanotubes in Design of Active SERS Substrates for High Sensitivity Analytical Applications: Effect of Geometrical Factors in Nanotubes and in Ag-n Deposits. In Raman Spectroscopy; InTech: London, UK, 2018. [Google Scholar]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisarek, M.; Ambroziak, R.; Hołdyński, M.; Roguska, A.; Majchrowicz, A.; Wysocki, B.; Kudelski, A. Nanofunctionalization of Additively Manufactured Titanium Substrates for Surface-Enhanced Raman Spectroscopy Measurements. Materials 2022, 15, 3108. https://doi.org/10.3390/ma15093108
Pisarek M, Ambroziak R, Hołdyński M, Roguska A, Majchrowicz A, Wysocki B, Kudelski A. Nanofunctionalization of Additively Manufactured Titanium Substrates for Surface-Enhanced Raman Spectroscopy Measurements. Materials. 2022; 15(9):3108. https://doi.org/10.3390/ma15093108
Chicago/Turabian StylePisarek, Marcin, Robert Ambroziak, Marcin Hołdyński, Agata Roguska, Anna Majchrowicz, Bartłomiej Wysocki, and Andrzej Kudelski. 2022. "Nanofunctionalization of Additively Manufactured Titanium Substrates for Surface-Enhanced Raman Spectroscopy Measurements" Materials 15, no. 9: 3108. https://doi.org/10.3390/ma15093108
APA StylePisarek, M., Ambroziak, R., Hołdyński, M., Roguska, A., Majchrowicz, A., Wysocki, B., & Kudelski, A. (2022). Nanofunctionalization of Additively Manufactured Titanium Substrates for Surface-Enhanced Raman Spectroscopy Measurements. Materials, 15(9), 3108. https://doi.org/10.3390/ma15093108