Heat Treatment of NiTi Alloys Fabricated Using Laser Powder Bed Fusion (LPBF) from Elementally Blended Powders
Abstract
:1. Introduction
2. Materials and Methods
3. Results
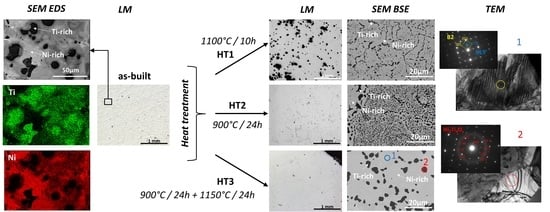
3.1. Microscopic Observation and Phase Analysis
3.2. Differential Thermal Analysis
3.3. Transmission Electron Microscopy
3.4. Oxygen Content
4. Discussion
4.1. As-Built Sample
4.2. First Heat Treatment—HT1
4.3. Second Heat Treatment—HT2
4.4. Third Heat Treatment—HT3
5. Conclusions
- The selected two-step HT3 heat treatment condition (900 °C/24 h + 1150 °C/24 h) allows for the significant homogenization of the chemical and phase composition of the LPBF in situ alloyed NiTi components;
- The Ti-rich phases in the as-built material melt during the chosen HT1 temperature (1100 °C) and upon solidification shrinkage occurs resulting in pore formation;
- Oxygen pickup during the LBPF manufacturing process promoted the formation of a thermodynamically stable, oxygen-rich Ni2Ti4O phase that is observed even after an annealing heat treatment;
- LPBF combined with post annealing is a promising way of fabricating NiTi alloys using elemental powder blends. Elimination of the oxygen pickup during the process and decrease of the possibility for formation of the oxides like Ni2Ti4O could be reached by undergoing the process in vacuum conditions, however further studies proving this hypothesis should be performed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgson, D.E.; Wu, M.H.; Biermann, R.J. Shape Memory Alloys; ASM International: Novelty, OH, USA, 2013; Volume 2. [Google Scholar]
- Elahinia, M.; Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Bimber, B.A.; Hamilton, R.F. Fabrication of NiTi through additive manufacturing: A review. Prog. Mater. Sci. 2016, 83, 630–663. [Google Scholar] [CrossRef] [Green Version]
- Greiner, C.; Oppenheimer, S.M.; Dunand, D.C. High strength, low stiffness, porous NiTi with superelastic properties. Acta Biomater. 2005, 1, 705–716. [Google Scholar] [CrossRef]
- Duering, T.W.; Pelton, A.R. Materials Properties Handbook: Titanium Alloys, 1st ed.; Welsch, G., Boyer, R., Collings, E.W., Eds.; ASM International the Materials Information Society: Materials Park, OH, USA, 1994; ISBN 978-0-87170-481-8. [Google Scholar]
- Kaynak, Y. Machining and phase transformation response of room-temperature austenitic NiTi shape memory alloy. J. Mater. Eng. Perform. 2014, 23, 3354–3360. [Google Scholar] [CrossRef]
- Kaynak, Y.; Karaca, H.E.; Noebe, R.D.; Jawahir, I.S. Analysis of Tool-wear and Cutting Force Components in Dry, Preheated, and Cryogenic Machining of NiTi Shape Memory Alloys. Procedia CIRP 2013, 8, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kustov, S.; Van Humbeeck, J. A short review on the microstructure, transformation behavior and functional properties of NiTi shape memory alloys fabricated by selective laser melting. Materials 2018, 11, 1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber, E.; Zhu, J.N.; Popovich, A.; Popovich, V. A review of NiTi shape memory alloy as a smart material produced by additive manufacturing. Mater. Today Proc. 2019, 30, 761–767. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Liu, Y.; An, J.; Chua, C.K.; Shen, Y.F.; Kuo, C.N. A review of selective laser melted NiTi shape memory alloy. Materials 2018, 11, 519. [Google Scholar] [CrossRef] [Green Version]
- Chmielewska, A.; Wysocki, B.; Buhagiar, J.; Michalski, B.; Adamczyk-Cieślak, B.; Gloc, M.; Święszkowski, W. In situ alloying of NiTi: Influence of Laser Powder Bed Fusion (LBPF) scanning strategy on chemical composition. Mater. Today Commun. 2022, 30, 103007. [Google Scholar] [CrossRef]
- Speirs, M.; Wang, X.; Van Baelen, S.; Ahadi, A.; Dadbakhsh, S.; Kruth, J.-P.; Van Humbeeck, J. On the Transformation Behavior of NiTi Shape-Memory Alloy Produced by SLM. Shape Mem. Superelasticity 2016, 2, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Bormann, T.; Müller, B.; Schinhammer, M.; Kessler, A.; Thalmann, P.; De Wild, M. Microstructure of selective laser melted nickel-titanium. Mater. Charact. 2014, 94, 189–202. [Google Scholar] [CrossRef]
- Frenzel, J.; George, E.P.; Dlouhy, A.; Somsen, C.; Wagner, M.F.X.; Eggeler, G. Influence of Ni on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2010, 58, 3444–3458. [Google Scholar] [CrossRef]
- Wang, C.; Tan, X.P.; Du, Z.; Chandra, S.; Sun, Z.; Lim, C.W.J.; Tor, S.B.; Lim, C.S.; Wong, C.H. Additive manufacturing of NiTi shape memory alloys using pre-mixed powders. J. Mater. Process. Technol. 2019, 271, 152–161. [Google Scholar] [CrossRef]
- Stoll, P.; Spierings, A.; Wegner, K. SLM processing of elementally blended NiTi shape memory alloy. Procedia CIRP 2020, 95, 121–126. [Google Scholar] [CrossRef]
- Halani, P.R.; Shin, Y.C. In situ synthesis and characterization of shape memory alloy nitinol by laser direct deposition. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 650–657. [Google Scholar] [CrossRef]
- Zhao, C.; Liang, H.; Luo, S.; Yang, J.; Wang, Z. The effect of energy input on reaction, phase transition and shape memory effect of NiTi alloy by selective laser melting. J. Alloys Compd. 2020, 817, 153288. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Coddet, C. Microstructure and transformation behavior of in-situ shape memory alloys by selective laser melting Ti-Ni mixed powder. J. Mater. Sci. Technol. 2013, 29, 863–867. [Google Scholar] [CrossRef]
- Mosallanejad, M.H.; Niroumand, B.; Aversa, A.; Saboori, A. In-situ alloying in laser-based additive manufacturing processes: A critical review. J. Alloys Compd. 2021, 872, 159567. [Google Scholar] [CrossRef]
- Katz-Demyanetz, A.; Koptyug, A.; Popov, V.V. In-situ Alloying as a Novel Methodology in Additive Manufacturing. In Proceedings of the 2020 IEEE 10th International Conference Nanomaterials: Applications & Properties (NAP), Sumy, Ukraine, 9–13 November 2020; pp. 26–29. [Google Scholar] [CrossRef]
- Li, S.; Hassanin, H.; Attallah, M.M.; Adkins, N.J.E.; Essa, K. The development of TiNi-based negative Poisson’s ratio structure using selective laser melting. Acta Mater. 2016, 105, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Shin, Y.C. Effects of Composition and Post Heat Treatment on Shape Memory Characteristics and Mechanical Properties for Laser Direct Deposited Nitinol. Lasers Manuf. Mater. Process. 2019, 6, 41–58. [Google Scholar] [CrossRef]
- Ibrahim, H.; Dean, D.; Klarner, A.D.; Luo, A.A. The Effect of Heat-Treatment on Mechanical, Microstructural, and Corrosion Characteristics of a Magnesium Alloy with Potential Application in Resorbable Bone Fixation Hardware. In Proceedings of the ASME 2016 11th International Manufacturing Science and Engineering Conference, MSEC2016-8822, Blacksburg, VA, USA, 27 June–1 July 2016; pp. 1–7. [Google Scholar]
- Shiva, S.; Palani, I.A.; Mishra, S.K.; Paul, C.P.; Kukreja, L.M. Investigations on the influence of composition in the development of Ni-Ti shape memory alloy using laser based additive manufacturing. Opt. Laser Technol. 2015, 69, 44–51. [Google Scholar] [CrossRef]
- Halani, P.R.; Kaya, I.; Shin, Y.C.; Karaca, H.E. Phase transformation characteristics and mechanical characterization of nitinol synthesized by laser direct deposition. Mater. Sci. Eng. A 2013, 559, 836–843. [Google Scholar] [CrossRef]
- Shishkovsky, I.; Yadroitsev, I.; Smurov, I. Direct Selective Laser Melting of Nitinol Powder. Phys. Procedia 2012, 39, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Chekotu, J.C.; Groarke, R.; O’Toole, K.; Brabazon, D. Advances in selective laser melting of Nitinol shape memory alloy part production. Materials 2019, 12, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielewska, A.; Jahadakbar, A.; Wysocki, B.; Elahinia, M.; Święszkowski, W.; Dean, D. Chemical Polishing of Additively Manufactured, Porous, Nickel–Titanium Skeletal Fixation Plates. 3D Print. Addit. Manuf. 2021. ahead of print. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Guo, W.; Guan, Y.; Wang, Z.; Zeng, Q.; Wang, X. Improved superelastic stability of NiTi shape memory alloys through surface nano-crystallization followed by low temperature aging treatment. Intermetallics 2021, 131, 8. [Google Scholar] [CrossRef]
- Divinski, S.V.; Stloukal, I.; Kral, L.; Herzig, C. Diffusion of titanium and nickel in B2 NiTi. Defect Diffus. Forum 2009, 289–292, 377–382. [Google Scholar] [CrossRef]
- Wilkinson, D.S. Mass Transport in Solids and Fluids; Cambridge University Press: Cambridge, UK, 2000; ISBN 0521624940. [Google Scholar]
- Motemani, Y.; Nili-Ahmadabadi, M.; Tan, M.J.; Bornapour, M.; Rayagan, S. Effect of cooling rate on the phase transformation behavior and mechanical properties of Ni-rich NiTi shape memory alloy. J. Alloys Compd. 2009, 469, 164–168. [Google Scholar] [CrossRef]
- Thomas, F. The Effect of Various Quenchants on the Hardness and Microstructure of 60-NITINOL; Nasa/TM-2015-218463; Glenn Research Center: Cleveland, OH, USA, 2015.
- Chen, K.C. NiTi—Magic or phase transformations? In Proceedings of the ASEE Annual Conference & Exposition, Nashville, TN, USA, 21 June 2003; pp. 2423–2430. [Google Scholar]
- Sitepu, H. Texture and structural refinement using neutron diffraction data from molybdite (MoO3) and calcite (CaCO3) powders and a Ni-rich Ni50.7Ti49.30 alloy. Powder Diffr. 2009, 24, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Kudoh, Y.; Tokonami, M.; Miyazaki, S.; Otsuka, K. Crystal structure of the martensite in Ti-49.2 at.%Ni alloy analyzed by the single crystal X-ray diffraction method. Acta Metall. 1985, 33, 2049–2056. [Google Scholar] [CrossRef]
- Mueller, M.H.; Knott, H.W. The crystal structures of Ti2Cu, Ti2Ni, Ti4Ni2O, and Ti4Cu2O. Trans. Metall. Soc. Aime 1963, 227, 674–678. [Google Scholar]
- Laves, F.; Wallbaum, J.H. Die Kristallstruktur von Ni3Ti und Si3Ti. Z. Für Krist.—Cryst. Mater. 1939, 101, 78–93. [Google Scholar] [CrossRef]
- Tirry, W.; Schryvers, D.; Jorissen, K.; Lamoen, D. Electron-diffraction structure refinement of Ni4Ti3 precipitates in Ni52Ti48. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, J.; Rogal, Ł.; Kalita, D.; Węglowski, M.; Błacha, S.; Berent, K.; Czeppe, T.; Antolak-Dudka, A.; Durejko, T.; Czujko, T. Superelastic Effect in NiTi Alloys Manufactured Using Electron Beam and Focused Laser Rapid Manufacturing Methods. J. Mater. Eng. Perform. 2020, 29, 4463–4473. [Google Scholar] [CrossRef]
- Morris, D.G.; Morris, M.A. NiTi intermetallic by mixing, milling and interdiffusing elemental components. Mater. Sci. Eng. A 1989, 110, 139–149. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Levitas, V.I. Internal stress-induced melting below melting temperature at high-rate laser heating. Appl. Phys. Lett. 2014, 104, 263106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cheng, X.; Cai, H. Fabrication, characterization and tensile property of a novel Ti2Ni/TiNi micro-laminated composite. Mater. Des. 2016, 92, 486–493. [Google Scholar] [CrossRef]
- Povoden-Karadeniz, E.; Cirstea, D.C.; Lang, P.; Wojcik, T.; Kozeschnik, E. Thermodynamics of Ti-Ni shape memory alloys. Calphad 2013, 41, 128–139. [Google Scholar] [CrossRef]
- Kai, W.Y.; Chang, K.C.; Wu, H.F.; Chen, S.W.; Yeh, A.C. Formation mechanism of Ni2Ti4Ox in NITI shape memory alloy. Materialia 2019, 5, 100194. [Google Scholar] [CrossRef]
- Takeshita, H.T.; Tanaka, H.; Kuriyama, N.; Sakai, T.; Uehara, I.; Haruta, M. Hydrogenation characteristics of ternary alloys containing Ti4Ni2X (X = O, N, C). J. Alloys Compd. 2000, 311, 188–193. [Google Scholar] [CrossRef]
- Karlík, M.; Haušild, P.; Klementová, M.; Novák, P.; Beran, P.; Perrière, L.; Kopeček, J. TEM phase analysis of NiTi shape memory alloy prepared by self-propagating high-temperature synthesis. Adv. Mater. Process. Technol. 2017, 3, 58–69. [Google Scholar] [CrossRef]
- Chmielewska, A.; Dobkowska, A.; Kijeska-Gawrońska, E.; Jakubczak, M.; Krawczyńska, A.; Choińska, E.; Jastrzębska, A.; Dean, D.; Wysocki, B.; Święszkowski, W. Biological and Corrosion Evaluation of In Situ Alloyed NiTi Fabricated through Laser Powder Bed Fusion (LPBF). Int. J. Mol. Sci. 2021, 22, 13209. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Kaserer, L.; Bergmueller, S.; Braun, J.; Leichtfried, G. Vacuum laser powder bed fusion—track consolidation, powder denudation, and future potential. Int. J. Adv. Manuf. Technol. 2020, 110, 3339–3346. [Google Scholar] [CrossRef]
- Cooper, N.; Coles, L.A.; Everton, S.; Maskery, I.; Campion, R.P.; Madkhaly, S.; Morley, C.; O’Shea, J.; Evans, W.; Saint, R.; et al. Additively manufactured ultra-high vacuum chamber for portable quantum technologies. Addit. Manuf. 2021, 40, 101898. [Google Scholar] [CrossRef]
- Martins, J.N.R.; Silva, E.J.N.L.; Marques, D.; Belladonna, F.; Simões-Carvalho, M.; Vieira, V.T.L.; Antunes, H.S.; Braz Fernandes, F.M.B.; Versiani, M.A. Design, metallurgical features, mechanical performance and canal preparation of six reciprocating instruments. Int. Endod. J. 2021, 54, 1623–1637. [Google Scholar] [CrossRef]








| Name | Number of Steps | Temperature [°C] | Time [h] |
|---|---|---|---|
| HT1 | 1 | 1100 | 10 |
| HT2 | 1 | 900 | 24 |
| HT3 | 2 | 900 + 1150 | 24 + 24 |
| HT1 | HT2 | HT3 | |
|---|---|---|---|
| NiTi2/Ni2Ti4O | 18 | 26 | 12.5 |
| volume fraction (%) | ±2 | ±4 | ±0.5 |
| As-Built | HT1 | HT2 | HT3 | |
|---|---|---|---|---|
| oxygen content (wt.%) | 0.52 ±0.1 | 0.53 ±0.1 | 0.54 ±0.2 | 0.52 ±0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewska, A.; Wysocki, B.; Kwaśniak, P.; Kruszewski, M.J.; Michalski, B.; Zielińska, A.; Adamczyk-Cieślak, B.; Krawczyńska, A.; Buhagiar, J.; Święszkowski, W. Heat Treatment of NiTi Alloys Fabricated Using Laser Powder Bed Fusion (LPBF) from Elementally Blended Powders. Materials 2022, 15, 3304. https://doi.org/10.3390/ma15093304
Chmielewska A, Wysocki B, Kwaśniak P, Kruszewski MJ, Michalski B, Zielińska A, Adamczyk-Cieślak B, Krawczyńska A, Buhagiar J, Święszkowski W. Heat Treatment of NiTi Alloys Fabricated Using Laser Powder Bed Fusion (LPBF) from Elementally Blended Powders. Materials. 2022; 15(9):3304. https://doi.org/10.3390/ma15093304
Chicago/Turabian StyleChmielewska, Agnieszka, Bartłomiej Wysocki, Piotr Kwaśniak, Mirosław Jakub Kruszewski, Bartosz Michalski, Aleksandra Zielińska, Bogusława Adamczyk-Cieślak, Agnieszka Krawczyńska, Joseph Buhagiar, and Wojciech Święszkowski. 2022. "Heat Treatment of NiTi Alloys Fabricated Using Laser Powder Bed Fusion (LPBF) from Elementally Blended Powders" Materials 15, no. 9: 3304. https://doi.org/10.3390/ma15093304
APA StyleChmielewska, A., Wysocki, B., Kwaśniak, P., Kruszewski, M. J., Michalski, B., Zielińska, A., Adamczyk-Cieślak, B., Krawczyńska, A., Buhagiar, J., & Święszkowski, W. (2022). Heat Treatment of NiTi Alloys Fabricated Using Laser Powder Bed Fusion (LPBF) from Elementally Blended Powders. Materials, 15(9), 3304. https://doi.org/10.3390/ma15093304