Physicochemical Characterization of Novel Epoxidized Vegetable Oil from Chia Seed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
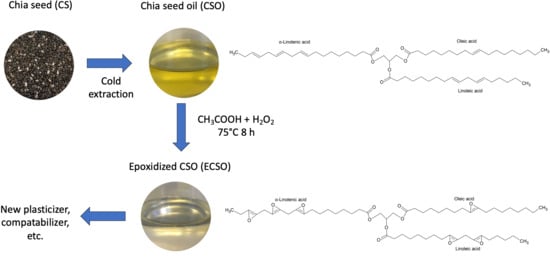
2.2. Epoxidation Process
2.3. Characterization
2.3.1. Fatty Acid Composition
2.3.2. Iodine Value (IV)
2.3.3. Oxirane Oxygen Content (Oo)
2.3.4. Epoxy Equivalent Weight (EEW)
2.3.5. Fourier Transform InfraRed (FTIR) Spectroscopy
2.3.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.7. Physico-Chemical Properties
3. Results
3.1. CSO Extraction and Fatty Acid Composition
3.2. Effect of Molar Ratio H2O2:Double Bond in the Epoxidation Process
3.3. Effect of the Temperature in the Epoxidation Process
3.4. FTIR Analysis
3.5. 1H NMR Analysis
3.6. Physico-Chemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| FTIR | Fourier Transform InfraRed spectroscopy | PUFA | polyunsaturated fatty acid |
| atomic mass iodine (126.9 g/mol) | S | selectivity | |
| atomic mass oxygen (16 g/mol) | SFA | saturated fatty acid | |
| volume HBr for titration of blank (mL) | temperature (K) | ||
| normality thiosulfate (N) | volume HBr for titration of sample (mL) | ||
| CSO | chia seed oil | volume thiosulfate for titration blank (mL) | |
| EEW | epoxy equivalent weight (g·eq−1) | volume thiosulfate for titration of sample (mL) | |
| EVO | epoxidized vegetable oil | VO | vegetable oil |
| ECSO | epoxidized chia seed oil | amount of sample for epoxy content (g) | |
| FA | fatty acids | weight of empty pycnometer (g) | |
| FAME | fatty acids methyl esters | weight of the sample in pycnometer (g) | |
| GC | gas chromatography | weight of water in pycnometer (g) | |
| iodine value | conversion of iodine value (%) | ||
| initial iodine value (g I2/100 g oil) | oxirane oxygen yield (%) | ||
| final iodine value (g I2/100 g oil) | |||
| m | amount of sample in iodine value (g) | Greek letters | |
| MR | molar ratio H2O2 to double bond | a | difference of green to red (a*) |
| MUFA | monounsaturated fatty acid | b | difference of blue to yellow (b*) |
| normality of HBr (N) | E | color variation | |
| oxirane oxygen content (wt.%) | L | difference of luminance (L*) | |
| theorical maximum oxirane oxygen (%) | specific gravity | ||
References
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Bioplastics, E. Bioplastics Market Data. Available online: https://www.european-bioplastics.org/ (accessed on 19 April 2022).
- Danov, S.M.; Kazantsev, O.A.; Esipovich, A.L.; Belousov, A.S.; Rogozhin, A.E.; Kanakov, E.A. Recent advances in the field of selective epoxidation of vegetable oils and their derivatives: A review and perspective. Catal. Sci. Technol. 2017, 7, 3659–3675. [Google Scholar] [CrossRef]
- Mungroo, R.; Pradhan, N.C.; Goud, V.V.; Dalai, A.K. Epoxidation of Canola Oil with Hydrogen Peroxide Catalyzed by Acidic Ion Exchange Resin. J. Am. Oil Chem. Soc. 2008, 85, 887–896. [Google Scholar] [CrossRef]
- Chieng, B.; Ibrahim, N.; Then, Y.; Loo, Y. Epoxidized Vegetable Oils Plasticized Poly (lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Development of toughened bio-based epoxy with epoxidized linseed oil as reactive diluent and cured with bio-renewable crosslinker. Polym. Adv. Technol. 2018, 29, 565–574. [Google Scholar] [CrossRef]
- Tee, Y.B.; Talib, R.A.; Abdan, K.; Chin, N.L.; Kadir Basha, R.; Md Yunos, K.F. Comparative Study of Chemical, Mechanical, Thermal, and Barrier Properties of Poly (Lactic Acid) Plasticized with Epoxidized Soybean Oil and Epoxidized Palm Oil. Bioresources 2015, 11, 1518–1540. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Yang, Y.; Feng, J.; Zhang, X.; Zhang, C.; Tang, Z.; Zhu, J. Preparation and characterization of poly (lactic acid)/starch composites toughened with epoxidized soybean oil. Carbohydr. Polym. 2013, 92, 810–816. [Google Scholar] [CrossRef]
- Janković, M.R.; Govedarica, O.M.; Sinadinović-Fišer, S.V. The epoxidation of linseed oil with in situ formed peracetic acid: A model with included influence of the oil fatty acid composition. Ind. Crops Prod. 2020, 143, 111881. [Google Scholar] [CrossRef]
- Carbonell-Verdu, A.; Bernardi, L.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Development of environmentally friendly composite matrices from epoxidized cottonseed oil. Eur. Polym. J. 2015, 63, 1–10. [Google Scholar] [CrossRef]
- Derawi, D.; Salimon, J. Optimization on Epoxidation of Palm Olein by Using Performic Acid. Eur. J. Chem. 2010, 7, 1440–1448. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.P.; Loppez-Martinez, J.; Samper, M.D. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Development of completely bio-based epoxy networks derived from epoxidized linseed and castor oil cured with citric acid. Polym. Adv. Technol. 2018, 29, 2080–2090. [Google Scholar] [CrossRef]
- Vianello, C.; Piccolo, D.; Lorenzetti, A.; Salzano, E.; Maschio, G. Study of Soybean Oil Epoxidation: Effects of Sulfuric Acid and the Mixing Program. Ind. Eng. Chem. Res. 2018, 57, 11517–11525. [Google Scholar] [CrossRef]
- Zheng, J.L.; Wärnå, J.; Salmi, T.; Burel, F.; Taouk, B.; Leveneur, S. Kinetic modeling strategy for an exothermic multiphase reactor system: Application to vegetable oils epoxidation using P rileschajew method. AIChE J. 2016, 62, 726–741. [Google Scholar] [CrossRef]
- Cai, X.; Ait Aissa, K.; Estel, L.; Leveneur, S. Investigation of the physicochemical properties for vegetable oils and their epoxidized and carbonated derivatives. J. Chem. Eng. Data 2018, 63, 1524–1533. [Google Scholar] [CrossRef]
- Shuttleworth, P.S.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Flexible bionanocomposites from epoxidized hemp seed oil thermosetting resin reinforced with halloysite nanotubes. J. Phys. Chem. B 2017, 121, 2454–2467. [Google Scholar] [CrossRef]
- Chia Seeds Market Size Worth $4.7 Billion by 2025. Available online: https://www.grandviewresearch.com/press-release/global-chia-seeds-market (accessed on 15 April 2022).
- Shen, Y.; Zheng, L.; Jin, J.; Li, X.; Fu, J.; Wang, M.; Guan, Y.; Song, X. Phytochemical and biological characteristics of mexican chia seed oil. Molecules 2018, 23, 3219. [Google Scholar] [CrossRef] [Green Version]
- Ullah, R.; Nadeem, M.; Imran, M.; Taj Khan, I.; Shahbaz, M.; Mahmud, A.; Tayyab, M. Omega fatty acids, phenolic compounds, and lipolysis of cheddar cheese supplemented with chia (Salvia hispanica L.) oil. J. Food Process. Preserv. 2018, 42, e13566. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Taj, I.; Ajmal, M.; Junaid, M. Omega-3 fatty acids, phenolic compounds and antioxidant characteristics of chia oil supplemented margarine. Lipids Health Dis. 2017, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Candela, I.; Ferri, J.M.; Cardona, S.C.; Lora, J.; Fombuena, V. Dual plasticizer/thermal stabilizer effect of epoxidized chia seed oil (Salvia hispanica L.) to improve ductility and thermal properties of poly (lactic acid). Polymers 2021, 13, 1283. [Google Scholar] [CrossRef]
- Warwel, S. Complete and partial epoxidation of plant oils by lipase-catalyzed perhydrolysis. Ind. Crops Prod. 1999, 9, 125–132. [Google Scholar]
- Biermann, U.; Friedt, W.; Lang, S.; Lühs, W.; Machmüller, G.; Metzger, J.O.; Rüsch gen. Klaas, M.; Schäfer, H.J.; Schneider, M.P. New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 2000, 39, 2206–2224. [Google Scholar] [CrossRef]
- Wallace, J.C. Encyclopedia of Chemical Technology, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1978; Volume 9. [Google Scholar]
- de Quadros, J.V.; Giudici, R. Epoxidation of soybean oil at maximum heat removal and single addition of all reactants. Chem. Eng. Process. Process Intensif. 2016, 100, 87–93. [Google Scholar] [CrossRef]
- Okieimen, F.E.; Bakare, O.I.; Okieimen, C.O. Studies on the epoxidation of rubber seed oil. Ind. Crops Prod. 2002, 15, 139–144. [Google Scholar] [CrossRef]
- Otabor, G.O.; Ifijen, I.H.; Mohammed, F.U.; Aigbodion, A.I.; Ikhuoria, E.U. Alkyd resin from rubber seed oil/linseed oil blend: A comparative study of the physiochemical properties. Heliyon 2019, 5, e01621. [Google Scholar] [CrossRef] [Green Version]
- Benaniba, M.; Belhaneche-Bensemra, N.; Gelbard, G. Epoxidation of sunflower oil with peroxoacetic acid in presence of ion exchange resin by various processes. Energ. Educ. Sci. Technol. 2008, 21, 71. [Google Scholar]
- Huang, Y.B.; Yao, M.Y.; Xin, P.P.; Zhou, M.C.; Yang, T.; Pan, H. Influence of alkenyl structures on the epoxidation of unsaturated fatty acid methyl esters and vegetable oils. RSC Adv. 2015, 5, 74783–74789, (restar uno a todo a partir de aquí). [Google Scholar] [CrossRef]
- Sun, S.; Yang, G.; Bi, Y.; Liang, H. Enzymatic epoxidation of corn oil by perstearic acid. J. Am. Oil Chem. Soc. 2011, 88, 1567–1571. [Google Scholar] [CrossRef]
- Sienel, G.; Rieth, R.; Rowbottom, K.T. Epoxides. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley & Sons: Hoboken, NJ, USA, 2003; Volume 12, pp. 269–284. [Google Scholar]
- Yadav, G.; Satoskar, D. Kinetics of epoxidation of alkyl esters of undecylenic acid: Comparison of traditional routes vs. Ishii-Venturello chemistry. J. Am. Oil Chem. Soc. 1997, 74, 397–407. [Google Scholar] [CrossRef]
- Leveneur, S. Thermal safety assessment through the concept of structure–reactivity: Application to vegetable oil valorization. Org. Process Res. Dev. 2017, 21, 543–550. [Google Scholar] [CrossRef]
- Moreno, V.C.; Russo, V.; Tesser, R.; Di Serio, M.; Salzano, E. Thermal risk in semi-batch reactors: The epoxidation of soybean oil. Process Saf. Environ. Prot. 2017, 109, 529–537. [Google Scholar] [CrossRef]
- Tan, S.; Chow, W. Biobased epoxidized vegetable oils and its greener epoxy blends: A review. Polym.-Plast. Technol. Eng. 2010, 49, 1581–1590. [Google Scholar] [CrossRef]
- Cai, X.; Zheng, J.L.; Aguilera, A.F.; Vernières–Hassimi, L.; Tolvanen, P.; Salmi, T.; Leveneur, S. Influence of ring-opening reactions on the kinetics of cottonseed oil epoxidation. Int. J. Chem. Kinet. 2018, 50, 726–741. [Google Scholar] [CrossRef]
- Jadhav, P.D.; Patwardhan, A.V.; Kulkarni, R.D. Kinetic study of in situ epoxidation of mustard oil. Mol. Catal. 2021, 511, 111748. [Google Scholar] [CrossRef]
- Sienkiewicz, A.M.; Czub, P. The unique activity of catalyst in the epoxidation of soybean oil and following reaction of epoxidized product with bisphenol A. Ind. Crops Prod. 2016, 83, 755–773. [Google Scholar] [CrossRef]
- Di Serio, M.; Russo, V.; Santacesaria, E.; Tesser, R.; Turco, R.; Vitiello, R. Liquid–liquid–solid model for the epoxidation of soybean oil catalyzed by Amberlyst-16. Ind. Eng. Chem. Res. 2017, 56, 12963–12971. [Google Scholar] [CrossRef]
- Wai, P.T.; Jiang, P.; Shen, Y.; Zhang, P.; Gu, Q.; Leng, Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019, 9, 38119–38136. [Google Scholar] [CrossRef] [Green Version]
- Goud, V.V.; Patwardhan, A.V.; Pradhan, N.C. Studies on the epoxidation of mahua oil (Madhumica indica) by hydrogen peroxide. Bioresour. Technol. 2006, 97, 1365–1371. [Google Scholar] [CrossRef]
- Dinda, S.; Patwardhan, A.V.; Goud, V.V.; Pradhan, N.C. Epoxidation of cottonseed oil by aqueous hydrogen peroxide catalysed by liquid inorganic acids. Bioresour. Technol. 2008, 99, 3737–3744. [Google Scholar] [CrossRef]
- Omonov, T.S.; Kharraz, E.; Curtis, J.M. The epoxidation of canola oil and its derivatives. RSC Adv. 2016, 6, 92874–92886. [Google Scholar] [CrossRef]
- Cai, C.; Dai, H.; Chen, R.; Su, C.; Xu, X.; Zhang, S.; Yang, L. Studies on the kinetics of in situ epoxidation of vegetable oils. Eur. J. Lipid Sci. Technol. 2008, 110, 341–346. [Google Scholar] [CrossRef]
- Pérez, J.E.; Haagenson, D.; Pryor, S.; Ulven, C.; Wiesenborn, D. Production and characterization of epoxidized canola oil. Trans. ASABE 2009, 52, 1289–1297. [Google Scholar] [CrossRef]
- Garea, S.-A.; Corbu, A.-C.; Deleanu, C.; Iovu, H. Determination of the epoxide equivalent weight (EEW) of epoxy resins with different chemical structure and functionality using GPC and 1H-NMR. Polym. Test. 2006, 25, 107–113. [Google Scholar] [CrossRef]
- Durbetaki, A.J. Direct Titration of Oxirane Oxygen with Hydrogen Bromide in Acetic Acid. Anal. Chem. 1956, 28, 2000–2001. [Google Scholar] [CrossRef]
- Kasote, D.M.; Badhe, Y.S.; Hegde, M.V. Effect of mechanical press oil extraction processing on quality of linseed oil. Ind. Crops Prod. 2013, 42, 10–13. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Martinez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.n.M.; Diehl, B.W.K.; Nolasco, S.M.; Tomas, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- La Scala, J.; Wool, R.P. Effect of FA composition on epoxidation kinetics of TAG. J. Am. Oil Chem. Soc. 2002, 79, 373–378. [Google Scholar] [CrossRef]
- Demin, M.; Rabrenovic, B.; Pezo, L.; Lalicic-Petronijevic, J. Influence of chia seeds (Salvia hispanica L.) and extra virgin olive oil addition on nutritional properties of salty crackers. J. Food Meas. Charact. 2020, 14, 378–387. [Google Scholar] [CrossRef]
- Ayerza, R. Oil content and fatty acid composition of chia (Salvia hispanica L.) from five northwestern locations in Argentina. J. Am. Oil Chem. Soc. 1995, 72, 1079–1081. [Google Scholar] [CrossRef]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michalowska, A. The Chemical Composition and Nutritional Value of Chia Seeds-Current State of Knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Nadeem, M.; Manzoor, M.F.; Javed, A.; Ali, Z.; Akhtar, M.N.; Ali, M.; Hussain, Y. Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 2016, 15, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef]
- Campanella, A.; Baltanás, M.A.; Capel-Sanchez, M.; Campos-Martin, J.; Fierro, J. Soybean oil epoxidation with hydrogen peroxide using an amorphous Ti/SiO2 catalyst. Green Chem. 2004, 6, 330–334. [Google Scholar] [CrossRef]
- Santacesaria, E.; Russo, V.; Tesser, R.; Turco, R.; Di Serio, M. Kinetics of performic acid synthesis and decomposition. Ind. Eng. Chem. Res. 2017, 56, 12940–12952, ((restar uno a partir de aquí). [Google Scholar] [CrossRef]
- Campanella, A.; Baltanás, M.A. Degradation of the oxirane ring of epoxidized vegetable oils in liquid–liquid heterogeneous reaction systems. Chem. Eng. J. 2006, 118, 141–152. [Google Scholar] [CrossRef]
- Gan, L.; Goh, S.; Ooi, K. Kinetic studies of epoxidation and oxirane cleavage of palm olein methyl esters. J. Am. Oil Chem. Soc. 1992, 69, 347–351. [Google Scholar] [CrossRef]
- Samper, M.D.; Petrucci, R.; Sanchez-Nacher, L.; Balart, R.; Kenny, J.M. Properties of composite laminates based on basalt fibers with epoxidized vegetable oils. Mater. Eng. 2015, 72, 9–15. [Google Scholar] [CrossRef]
- Fombuena, V.; Petrucci, R.; Dominici, F.; Jordá-Vilaplana, A.; Montanes, N.; Torre, L. Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers 2019, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Timilsena, Y.P.; Vongsvivut, J.; Tobin, M.J.; Adhikari, R.; Barrow, C.; Adhikari, B. Investigation of oil distribution in spray-dried chia seed oil microcapsules using synchrotron-FTIR microspectroscopy. Food Chem. 2019, 275, 457–466. [Google Scholar] [CrossRef]
- Goud, V.V.; Dinda, S.; Patwardhan, A.V.; Pradhan, N.C. Epoxidation of Jatropha (Jatropha curcas) oil by peroxyacids. Asia Pac. J. Chem. Eng. 2010, 5, 346–354. [Google Scholar] [CrossRef]
- Kim, J.R.; Sharma, S. The development and comparison of bio-thermoset plastics from epoxidized plant oils. Ind. Crops Prod. 2012, 36, 485–499. [Google Scholar] [CrossRef]
- DeHonor-Marquez, E.; Nieto Alarcón, J.F.; Vigueras Santiago, E.; Hernández López, S. Effective and Fast Epoxidation Reaction of Linseed Oil Using 50 wt% Hydrogen Peroxyde. Am. J. Chem. 2018, 8, 99–106. [Google Scholar]
- Farias, M.; Martinelli, M.; Bottega, D.P. Epoxidation of soybean oil using a homogeneous catalytic system based on a molybdenum (VI) complex. Appl. Catal. A Gen. 2010, 384, 213–219. [Google Scholar] [CrossRef]
- Harry-O’kuru, R.E.; Biresaw, G.; Tisserat, B.; Evangelista, R. Synthesis of Polyformate Esters of Vegetable Oils: Milkweed, Pennycress, and Soy. J. Lipids 2016, 2016, 3128604. [Google Scholar] [CrossRef] [Green Version]
- Uzunova, G.; Nikolova, K.; Perifanova, M.; Gentscheva, G.; Marudova, M.; Antova, G. Physicochemical characterization of chia (Salvia hispanica) seed oil from Argentina. Bulg. Chem. Commun. 2016, 48, 131–135. [Google Scholar]
- Wool, R.; Sun, X.S. Bio-Based Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. The study of epoxidized rapeseed oil used as a potential biodegradable lubricant. J. Am. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Monono, E.M.; Haagenson, D.M.; Wiesenborn, D.P. Characterizing the epoxidation process conditions of canola oil for reactor scale-up. Ind. Crops Prod. 2015, 67, 364–372. [Google Scholar] [CrossRef]
- Alarcon, R.T.; Gaglieri, C.; Lamb, K.J.; North, M.; Bannach, G. Spectroscopic characterization and thermal behavior of baru nut and macaw palm vegetable oils and their epoxidized derivatives. Ind. Crops Prod. 2020, 154, 112585. [Google Scholar] [CrossRef]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the Influence of the Reprocessing Cycles on the Final Properties of Polylactide Pieces Obtained by Injection Molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [Green Version]










| Vegetable Oil | Initial Iodine Value (IV0, g I2/100 g) | Theoretical Oxirane Oxygen Content (Oo, %) | References |
|---|---|---|---|
| Castor | 84 | 5.03 | [14] |
| Soybean | 126 | 7.36 | [27] |
| Rubber | 156 | 8.95 | [28] |
| Cottonseed | 107 | 6.32 | [11] |
| Linseed | 188 | 10.6 | [29] |
| Canola | 112 | 6.60 | [5] |
| Sunflower | 130 | 7.57 | [30] |
| Palm | 62 | 3.76 | [12] |
| Olive | 127 | 7.41 | [31] |
| Corn | 115 | 6.76 | [32] |
| Chia seed | 197 | 11.05 | Present study |
| Fatty Acids (FAs) | In This STUDY | Demin et al. [53] | Kulczyński et al. [55] | |
|---|---|---|---|---|
| SFAs | Myristic (C14:0) | 0.06 | 0.04 | 0.06 |
| Palmitic (C16:0) | 7.20 | 6.84 | 7.04 | |
| Stearic (C18:0) | 2.88 | 2.71 | 2.84 | |
| Arachidic (C20:0) | 0.55 | 0.28 | 0.02 | |
| Total SFAs | 10.7 | 9.87 | 9.96 | |
| MUFAs | Palmitoleic (C16:1) | 0.09 | 0.24 | 0.03 |
| Oleic (C18:1) | 4.32 | 6.17 | 7.3 | |
| Total MUFAs | 4.41 | 6.41 | 7.33 | |
| PUFAs | Linoleic (C18:2) | 15.8 | 18.6 | 18.9 |
| γ-Linolenic (C18:3) | 0.41 | n.m 1 | n.m 1 | |
| α-Linolenic (C18:3) | 68.6 | 64.4 | 63.8 | |
| Total PUFAs | 84.9 | 83 | 82.7 |
| Epoxidation | Temperature (°C) | MR 1 | IVf2 | XIV3 | Oo4 | EEW5 (g·eq−1) | YOO6 | S7 |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 0.75 | 0.94 | 0.11 | 8.9 | 1.88 | ||
| 2 | 60 | 1.50 | 0.41 | 0.12 | 4.94 | 1.07 | 1.92 | |
| 3 | 70 | 1.50 | 0.62 | 0.32 | 3.10 | 0.98 | 1.70 | |
| 4 | 75 | 1.50 | 1.52 | 0.75 | 0.11 | 2.39 | 0.78 | 1.79 |
| Sample | Specific | Dynamic Viscosity (mPa·s) | Colourimetric Coordinates | Colour | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| CSO | 2.2·10−3 | 0.45 | 0.30 | 0.35 | 0 | |
| ECSO MR 0.75 60 °C | 1.2·10−3 | 0.78 | 0.28 | 0.21 | 0.21 | 0.25 |
| ECSO MR 1.50 60 °C | 2.3·10−3 | 0.73 | 0.23 | 0.17 | 0.49 | 0.19 |
| ECSO MR 1.50 70 °C | 1.7·10−3 | 0.87 | 0.28 | 0.35 | 0.16 | 0.44 |
| ECSO MR 1.50 75 °C | 2.9·10−3 | 1.02 | 0.42 | 0.36 | 0.21 | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez-Candela, I.; Lerma-Canto, A.; Cardona, S.C.; Lora, J.; Fombuena, V. Physicochemical Characterization of Novel Epoxidized Vegetable Oil from Chia Seed Oil. Materials 2022, 15, 3250. https://doi.org/10.3390/ma15093250
Dominguez-Candela I, Lerma-Canto A, Cardona SC, Lora J, Fombuena V. Physicochemical Characterization of Novel Epoxidized Vegetable Oil from Chia Seed Oil. Materials. 2022; 15(9):3250. https://doi.org/10.3390/ma15093250
Chicago/Turabian StyleDominguez-Candela, Ivan, Alejandro Lerma-Canto, Salvador Cayetano Cardona, Jaime Lora, and Vicent Fombuena. 2022. "Physicochemical Characterization of Novel Epoxidized Vegetable Oil from Chia Seed Oil" Materials 15, no. 9: 3250. https://doi.org/10.3390/ma15093250
APA StyleDominguez-Candela, I., Lerma-Canto, A., Cardona, S. C., Lora, J., & Fombuena, V. (2022). Physicochemical Characterization of Novel Epoxidized Vegetable Oil from Chia Seed Oil. Materials, 15(9), 3250. https://doi.org/10.3390/ma15093250