Effect of Shear Stress during Processing on Structure, Morphology, and Properties of Isotactic Polypropylene Nucleated with Silsesquioxane-Based β-Nucleating Agent
Abstract
:1. Introduction
2. Experimental
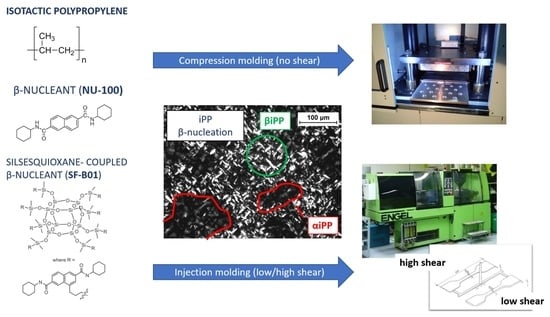
2.1. Materials and Sample Preparation
2.1.1. Materials and Methods
2.1.2. Sample Preparation
2.2. Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jubinville, D.; Esmizadeh, E.; Tzoganakis, C.; Mekonnen, T. Thermo-Mechanical Recycling of Polypropylene for the Facile and Scalable Fabrication of Highly Loaded Wood Plastic Composites. Compos. Part B Eng. 2021, 219, 108873. [Google Scholar] [CrossRef]
- Strömberg, E.; Karlsson, S. The Design of a Test Protocol to Model the Degradation of Polyolefins during Recycling and Service Life. J. Appl. Polym. Sci. 2009, 112, 1835–1844. [Google Scholar] [CrossRef]
- Plastics—The Facts 2022; Plastics Europe: Puteaux, France, 2022.
- Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. β-Nucleated Polypropylene: Processing, Properties and Nanocomposites. Polym. Rev. 2015, 55, 596–629. [Google Scholar] [CrossRef]
- Gahleitner, M. Are Polyolefins Outdated? Express Polym. Lett. 2020, 14, 704. [Google Scholar] [CrossRef]
- Bora, R.R.; Wang, R.; You, F. Waste Polypropylene Plastic Recycling toward Climate Change Mitigation and Circular Economy: Energy, Environmental, and Technoeconomic Perspectives. ACS Sustain. Chem. Eng. 2020, 8, 16350–16363. [Google Scholar] [CrossRef]
- Pukánszky, B.; Mudra, I.; Staniek, P. Relation of Crystalline Structure and Mechanical Properties of Nucleated Polypropylene. J. Vinyl Addit. Technol. 1997, 3, 53–57. [Google Scholar] [CrossRef]
- Avalos-Belmontes, F.; Ramos-deValle, L.F.; Espinoza-Martínez, A.B.; Martínez-Colunga, J.G.; Ramírez-Vargas, E.; Sánchez-Valdés, S.; Ortíz-Cisneros, J.C.; Martínez-Segovia, E.E.; Beltrán-Ramírez, F.I. Effect of Different Nucleating Agents on the Crystallization of Ziegler-Natta Isotactic Polypropylene. Int. J. Polym. Sci. 2016, 2016, 9839201. [Google Scholar] [CrossRef]
- Tenma, M.; Mieda, N.; Takamatsu, S.; Yamaguchi, M. Structure and Properties for Transparent Polypropylene Containing Sorbitol-Based Clarifier. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 41–47. [Google Scholar] [CrossRef]
- Tenma, M.; Yamaguchi, M. Structure and Properties of Injection-Molded Polypropylene with Sorbitol-Based Clarifier. Polym. Eng. Sci. 2007, 47, 1441–1446. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Li, Y.; Zhang, Y.; Xie, X.; Li, K.; Chen, Z.; Zhang, L.; Tang, Z.; Liu, Z. Nanoengineering of Transparent Polypropylene Containing Sorbitol-Based Clarifier. J. Polym. Res. 2020, 27, 198. [Google Scholar] [CrossRef]
- Farahani, M.; Jahani, Y. An Approach for Prediction Optimum Crystallization Conditions for Formation of Beta Polypropylene by Response Surface Methodology (RSM). Polym. Test. 2021, 93, 106921. [Google Scholar] [CrossRef]
- Luijsterburg, B.; Jobse, P.; Hermida Merino, D.; Peijs, T.; Goossens, H. Solid-State Drawing of β-Nucleated Polypropylene: Effect of Additives on Drawability and Mechanical Properties. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1071–1082. [Google Scholar] [CrossRef]
- Nitta, K.; Takashima, T. Tensile Properties in β-Modified Isotactic Polypropylene. In Polypropylene—Polymerization and Characterization of Mechanical and Thermal Properties; IntechOpen: London, UK, 2020. [Google Scholar]
- Romankiewicz, A.; Sterzynski, T.; Brostow, W. Structural characterization of α- and β-nucleated isotactic polypropylene. Polym. Int. 2004, 53, 2086–2091. [Google Scholar] [CrossRef]
- Obadal, M.; Čermák, R.; Raab, M.; Verney, V.; Commereuc, S.; Fraïsse, F. Structure Evolution of α- and β-Polypropylenes upon UV Irradiation: A Multiscale Comparison. Polym. Degrad. Stab. 2005, 88, 532–539. [Google Scholar] [CrossRef]
- Cho, K.; Nabi Saheb, D.; Yang, H.; Kang, B.-I.; Kim, J.; Lee, S.-S. Memory Effect of Locally Ordered α-Phase in the Melting and Phase Transformation Behavior of β-Isotactic Polypropylene. Polymer 2003, 44, 4053–4059. [Google Scholar] [CrossRef]
- Yoshida, H. Dynamic Analysis of the Melting Behavior of Polymers Showing Polymorphism Observed by Simultaneous DSC/X-Ray Diffraction Measurements. Thermochim. Acta 1995, 267, 239–248. [Google Scholar] [CrossRef]
- Dragaun, H.; Hubeny, H.; Muschik, H. Shear-Induced β-Form Crystallization in Isotactic Polypropylene. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 1779–1789. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Ji, F.; Zhang, X.; Zheng, G.; Shen, C. Effects of Melt Structure on Shear-Induced β-Cylindrites of Isotactic Polypropylene. Polymer 2012, 53, 1791–1800. [Google Scholar] [CrossRef]
- Bednarek, W.H.; Paukszta, D.; Szostak, M.; Szymańska, J. Fundamental Studies on Shear-Induced Nucleation and Beta-Phase Formation in the Isotactic Polypropylene—Effect of the Temperature. J. Polym. Res. 2021, 28, 439. [Google Scholar] [CrossRef]
- Yue, Y.; Feng, J. Structure Evolution upon Heating and Cooling and Its Effects on Nucleation Performance: A Review on Aromatic Amide Β-nucleating Agents for Isotactic Polypropylene. Polym. Cryst. 2019, 2, e10049. [Google Scholar] [CrossRef]
- Nie, S.; Zhong, J.-R.; Li, Y.; Zhang, Y.-F. Effect of Polyacrylic Salt Nucleating Agents on the Properties of Isotactic Polypropylene. J. Therm. Anal. Calorim. 2023. [Google Scholar] [CrossRef]
- Xiang, J.; Li, Y.; Zhong, J.-R.; Lu, C.-H.; Zhang, Y.-F. Influence of Chemical Structures of Bisamide Nucleating Agents on the Crystallization Behavior and Properties of Isotactic Polypropylene. J. Therm. Anal. Calorim. 2023, 148, 2417–2428. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Zhang, C.; Dong, Z.; Wang, K.; Wang, D. Morphological Characteristics of β-Nucleating Agents Governing the Formation of the Crystalline Structure of Isotactic Polypropylene. Macromolecules 2021, 54, 6824–6834. [Google Scholar] [CrossRef]
- Qin, W.; Liu, K.; Xin, Z.; Ling, H.; Zhou, S.; Zhao, S. Zinc Pimelate as an Effective β -nucleating Agent for Isotactic Polypropylene at Elevated Pressures and under Rapid Cooling Rates. Polym. Cryst. 2020, 3, e10132. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, L.; Zhang, Y.; Zhang, Y.; Yin, N. Isothermal Crystallization Kinetics of Polypropylene/POSS Composites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1762–1772. [Google Scholar] [CrossRef]
- Fu, B.X.; Yang, L.; Somani, R.H.; Zong, S.X.; Hsiao, B.S.; Phillips, S.; Blanski, R.; Ruth, P. Crystallization Studies of Isotactic Polypropylene Containing Nanostructured Polyhedral Oligomeric Silsesquioxane Molecules under Quiescent and Shear Conditions. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2727–2739. [Google Scholar] [CrossRef]
- Pracella, M.; Chionna, D.; Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene-POSS Nanocomposites: Morphology and Crystallization Behaviour. Macromol. Symp. 2006, 234, 59–67. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Dutkiewicz, M.; Marciniec, B. Study of Thermal Properties of Polyethylene and Polypropylene Nanocomposites with Long Alkyl Chain-Substituted POSS Fillers. J. Therm. Anal. Calorim. 2016, 125, 1287–1299. [Google Scholar] [CrossRef]
- Durmus, A.; Kasgoz, A.; Ercan, N.; Akın, D.; Şanlı, S. Effect of Polyhedral Oligomeric Silsesquioxane (POSS) Reinforced Polypropylene (PP) Nanocomposite on the Microstructure and Isothermal Crystallization Kinetics of Polyoxymethylene (POM). Polymer 2012, 53, 5347–5357. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene–Polyhedral Oligomeric Silsesquioxanes (POSS) Nanocomposites. Polymer 2005, 46, 7855–7866. [Google Scholar] [CrossRef]
- Herc, A.S.; Bojda, J.; Nowacka, M.; Lewiński, P.; Maniukiewicz, W.; Piorkowska, E.; Kowalewska, A. Crystallization, Structure and Properties of Polylactide/Ladder Poly(Silsesquioxane) Blends. Polymer 2020, 201, 122563. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Sterzynski, T. Effect of Polyhedral Oligomeric Silsesquioxane on the Melting, Structure, and Mechanical Behavior of Polyoxymethylene. Polymers 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.; Dziubek, K.; Czaja, K.; Szatanik, R.; Szolyga, M.; Dutkiewicz, M.; Marciniec, B. Polypropylene/Polyhedral Oligomeric Silsesquioxane Nanocomposites—Study of Free Volumes, Crystallinity Degree and Mass Flow Rate. Polimery 2016, 61, 610–615. [Google Scholar] [CrossRef]
- Barczewski, M.; Czarnecka-Komorowska, D.; Andrzejewski, J.; Sterzyński, T.; Dutkiewicz, M.; Dudziec, B. Processing Properties of Thermoplastic Polymers Modified by Polyhedral Oligomeric Silsesquioxanes (POSS). Polimery 2013, 58, 805–815. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Polypropylene-Based Composites Containing Sorbitol-Based Nucleating Agent and Siloxane-Silsesquioxane Resin. J. Appl. Polym. Sci. 2016, 133, 43476. [Google Scholar] [CrossRef]
- Mao, H.; Liu, Y.; Liu, W.; Nie, M.; Wang, Q. Investigation of Crystallisation and Interfacial Nature of Polyhedral Oligomeric Silsesquioxane/Polypropylene Composites in the Presence of β-Nucleating Agent. Plast. Rubber Compos. 2017, 46, 322–328. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Meng, X.; Xin, Z. The Mechanical Properties, Crystallization and Rheological Behavior of Isotactic Polypropylene with Nucleating Agent Supported on Polyhedral Oligomeric Silsesquioxanes (POSS). J. Polym. Res. 2020, 27, 303. [Google Scholar] [CrossRef]
- Roy, S.; Scionti, V.; Jana, S.C.; Wesdemiotis, C.; Pischera, A.M.; Espe, M.P. Sorbitol–POSS Interactions on Development of Isotactic Polypropylene Composites. Macromolecules 2011, 44, 8064–8079. [Google Scholar] [CrossRef]
- Roy, S.; Lee, B.J.; Kakish, Z.M.; Jana, S.C. Exploiting POSS–Sorbitol Interactions: Issues of Reinforcement of Isotactic Polypropylene Spun Fibers. Macromolecules 2012, 45, 2420–2433. [Google Scholar] [CrossRef]
- Barczewski, M.; Zdanowicz, M.; Mysiukiewicz, O.; Dobrzyńska-Mizera, M.; Dudziec, B. Effect of Tetrasilanolphenyl Silsesquioxane on Properties of Sorbitol Derivative-Nucleated Polypropylene Cast Films. Plast. Rubber Compos. 2023, 52, 204–215. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Barczewski, M.; Dudziec, B.; Sterzyñski, T. Influence of the Cooling Rate on the Non-Isothermal Crystallization of Isotactic Polypropylene Modified with Sorbitol Derivative and Silsesquioxane. Polimery 2013, 58, 920–923. [Google Scholar] [CrossRef]
- Barczewski, M.; Dobrzyńska-Mizera, M.; Dudziec, B.; Sterzyński, T. Influence of a Sorbitol-Based Nucleating Agent Modified with Silsesquioxanes on the Non-Isothermal Crystallization of Isotactic Polypropylene. J. Appl. Polym. Sci. 2014, 131, 40131. [Google Scholar] [CrossRef]
- Gao, J.; Cao, X.; Zhang, C.; Hu, W. Non-Isothermal Crystallization Kinetics of Polypropylene/MAP-POSS Nanocomposites. Polym. Bull. 2013, 70, 1977–1990. [Google Scholar] [CrossRef]
- Barczewski, M.; Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Szołyga, M. Novel Polypropylene β -Nucleating Agent with Polyhedral Oligomeric Silsesquioxane Core: Synthesis and Application. Polym. Int. 2016, 65, 1080–1088. [Google Scholar] [CrossRef]
- Chvátalová, L.; Navrátilová, J.; Čermák, R.; Raab, M.; Obadal, M. Joint Effects of Molecular Structure and Processing History on Specific Nucleation of Isotactic Polypropylene. Macromolecules 2009, 42, 7413–7417. [Google Scholar] [CrossRef]
- Menyhárd, A. Crystallization and Melting Characteristics and Supermolecular Structure of the β-Modification of Isotactic Polypropylene and Its Multi-Component Systems. Ph.D. Thesis, Budapest University of Technology and Economics, Budapest, Hungary, 2007. [Google Scholar]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral Oligomeric Silsesquioxanes (POSS) Thermal Degradation. Thermochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Banasiak, A.; Sterzyński, T. Assessment of a Flow of a Polymer, Filled with Lamellar Filler as a Marker, in an Injection Mold. Polimery 2004, 49, 442–448. [Google Scholar] [CrossRef]
- Li, J.X.; Cheung, W.L. On the Deformation Mechanisms of β-Polypropylene: 1. Effect of Necking on β-Phase PP Crystals. Polymer 1998, 39, 6935–6940. [Google Scholar] [CrossRef]
- Ding, C.; Wu, G.-G.; Zhang, Y.; Yang, Y.; Yin, B.; Yang, M.-B. Effect of Surfactant Assisted β-Nucleating Agent Self-Assembly on the Crystallization of Polypropylene. Polymer 2019, 184, 121895. [Google Scholar] [CrossRef]
- Varga, J.; Karger-Kocsis, J. Rules of Supermolecular Structure Formation in Sheared Isotactic Polypropylene Melts. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 657–670. [Google Scholar] [CrossRef]
- Bai, H.; Luo, F.; Zhou, T.; Deng, H.; Wang, K.; Fu, Q. New Insight on the Annealing Induced Microstructural Changes and Their Roles in the Toughening of β-Form Polypropylene. Polymer 2011, 52, 2351–2360. [Google Scholar] [CrossRef]
- Luo, B.; Li, H.; Zhang, W.; Zhou, C.; Li, J.; Lu, C.; He, X.; Jiang, S. Mechanistic Insights into the Shear Effects on Isotactic Polypropylene Crystallization Containing β-Nucleating Agent. Chinese J. Polym. Sci. 2017, 35, 672–680. [Google Scholar] [CrossRef]
- Ma, Y.; Xin, M.; Xu, K.; Chen, M. A Novel β-Nucleating Agent for Isotactic Polypropylene. Polym. Int. 2013, 62, 744–750. [Google Scholar] [CrossRef]
- Nezbedova, E.; Pospisil, V.; Bohaty, P.; Vlach, B. Fracture Behaviour Ofβ-Polypropylene as a Function of Processing Conditions. Macromol. Symp. 2001, 170, 349–357. [Google Scholar] [CrossRef]
- Huo, H.; Jiang, S.; An, L.; Feng, J. Influence of Shear on Crystallization Behavior of the β Phase in Isotactic Polypropylene with β-Nucleating Agent. Macromolecules 2004, 37, 2478–2483. [Google Scholar] [CrossRef]
- Stern, C.; Frick, A.; Weickert, G. Relationship between the Structure and Mechanical Properties of Polypropylene: Effects of the Molecular Weight and Shear-Induced Structure. J. Appl. Polym. Sci. 2007, 103, 519–533. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Yang, J.; Ding, D.; Yan, X.; Zheng, G.; Dai, K.; Liu, C.; Guo, Z. Synergies among the Self-Assembled β-Nucleating Agent and the Sheared Isotactic Polypropylene Matrix. Polymer 2015, 60, 40–49. [Google Scholar] [CrossRef]
- Pantani, R.; Coccorullo, I.; Volpe, V.; Titomanlio, G. Shear-Induced Nucleation and Growth in Isotactic Polypropylene. Macromolecules 2010, 43, 9030–9038. [Google Scholar] [CrossRef]
- Luo, F.; Wang, K.; Ning, N.; Geng, C.; Deng, H.; Chen, F.; Fu, Q.; Qian, Y.; Zheng, D. Dependence of Mechanical Properties on β-Form Content and Crystalline Morphology for β-Nucleated Isotactic Polypropylene. Polym. Adv. Technol. 2011, 22, 2044–2054. [Google Scholar] [CrossRef]
- Varga, J.; Menyhárd, A. Effect of Solubility and Nucleating Duality of N, N ‘-Dicyclohexyl-2,6-Naphthalenedicarboxamide on the Supermolecular Structure of Isotactic Polypropylene. Macromolecules 2007, 40, 2422–2431. [Google Scholar] [CrossRef]
- Karger-Kocis, J.; Friedrich, K. Effect of Skin-Core Morphology on Fatigue Crack Propagation in Injection Moulded Polypropylene Homopolymer. Int. J. Fatigue 1989, 11, 161–168. [Google Scholar] [CrossRef]
- Kersch, M.; Pischke, L.; Schmidt, H.-W.; Altstädt, V. Influence of Trisamide-Based Additives on the Morphological and Mechanical Properties of Isotactic Polypropylene. Polymer 2014, 55, 3227–3233. [Google Scholar] [CrossRef]














| Sample | Tmβ (°C) | Tmα (°C) | Tcr (°C) | Φ (%) | Xc (%) | |
|---|---|---|---|---|---|---|
| Compression molded | iPP | - | 166.7 | 117.6 | 0 | 39.2 |
| 0.01% NU-100 | 153.2 | 165.4 | 117.6 | 64.3 | 54.3 | |
| 0.05% NU-100 | 156.9 | 166.8 | 125.0 | 64.2 | 52.8 | |
| 0.10% NU-100 | 156.2 | 166.0 | 125.9 | 66.4 | 52.3 | |
| 0.25% NU-100 | 157.6 | 167.6 | 126.8 | 72.9 | 52.7 | |
| 0.50% NU-100 | 157.3 | 167.2 | 127.0 | 71.8 | 53.7 | |
| 0.01% SF-B01 | 152.8 | 165.9 | 118.7 | 69.5 | 55.7 | |
| 0.05% SF-B01 | 155.7 | 166.0 | 122.3 | 57.5 | 54.1 | |
| 0.10% SF-B01 | 156.9 | 167.1 | 125.6 | 59.5 | 53.8 | |
| 0.25% SF-B01 | 156.4 | 167.2 | 126.7 | 70.9 | 53.0 | |
| 0.50% SF-B01 | 152.5 | 166.2 | 127.2 | 60.3 | 48.8 | |
| Injection molded, 1 mm | iPP | - | 165.9 | 116.9 | 0 | 36.6 |
| 0.01% NU-100 | - | 165.8 | 117.7 | 0 | 47.4 | |
| 0.05% NU-100 | 150.1 | 165.2 | 125.3 | 7.9 | 47.6 | |
| 0.10% NU-100 | 150.0 | 166.5 | 126.4 | 17.9 | 47.8 | |
| 0.25% NU-100 | 150.5 | 166.4 | 127.0 | 18.1 | 47.8 | |
| 0.50% NU-100 | 150.6 | 166.2 | 127.5 | 19.5 | 41.1 | |
| 0.01% SF-B01 | - | 165.6 | 119.9 | 0 | 45.9 | |
| 0.05% SF-B01 | 150.4 | 166.5 | 124.3 | 13.0 | 41.5 | |
| 0.10% SF-B01 | 150.4 | 165.9 | 126.1 | 21.9 | 41.6 | |
| 0.25% SF-B01 | 150.7 | 166.2 | 126.6 | 17.4 | 42.0 | |
| 0.50% SF-B01 | 150.9 | 166.4 | 127.3 | 19.2 | 41.6 | |
| Injection molded, 3 mm | iPP | - | 167.9 | 116.9 | 0 | 36.6 |
| 0.01% NU-100 | - | 167.7 | 117.6 | 0 | 46.6 | |
| 0.05% NU-100 | 150.8 | 167.3 | 124.9 | 0.8 | 41.9 | |
| 0.10% NU-100 | 151.1 | 166.5 | 126.2 | 0.7 | 42.7 | |
| 0.25% NU-100 | - | 166.0 | 126.9 | 0 | 41.9 | |
| 0.50% NU-100 | - | 167.1 | 128.1 | 0 | 48.0 | |
| 0.01% SF-B01 | - | 167.0 | 117.9 | 0 | 43.3 | |
| 0.05% SF-B01 | - | 166.2 | 125.3 | 0 | 42.8 | |
| 0.10% SF-B01 | 151.2 | 166.4 | 124.9 | 2.2 | 40.3 | |
| 0.25% SF-B01 | - | 165.8 | 126.6 | 0 | 43.3 | |
| 0.50% SF-B01 | - | 167.0 | 127.4 | 0 | 42.9 |
| Sample | Tmβ [°C] | Tmα [°C] | Φ [%] | Xc [%] | |
|---|---|---|---|---|---|
| Compression molded | iPP | - | 162.8 | 0 | 39.2 |
| 0.01% NU-100 | - | 163.1 | 0 | 52.1 | |
| 0.05% NU-100 | 150.7 | 167.6 | 76.2 | 50.1 | |
| 0.10% NU-100 | 151.5 | 167.5 | 75.9 | 49.3 | |
| 0.25% NU-100 | 151.9 | 167.6 | 77.8 | 50.0 | |
| 0.50% NU-100 | 151.3 | 167.7 | 75.9 | 50.2 | |
| 0.01% SF-B01 | - | 162.8 | 0 | 52.7 | |
| 0.05% SF-B01 | 151.6 | 167.6 | 77.6 | 50.5 | |
| 0.10% SF-B01 | 151.9 | 167.5 | 76.3 | 50.8 | |
| 0.25% SF-B01 | 152.0 | 167.6 | 78.8 | 50.2 | |
| 0.50% SF-B01 | 152.5 | 167.7 | 73.7 | 48.6 | |
| Injection molded, 1 mm | iPP | - | 164.0 | 0 | 41.6 |
| 0.01% NU-100 | - | 163.8 | 0 | 53.3 | |
| 0.05% NU-100 | 151.6 | 167.0 | 70.9 | 55.0 | |
| 0.10% NU-100 | 151.8 | 167.7 | 73.8 | 55.1 | |
| 0.25% NU-100 | 152.2 | 167.6 | 71.1 | 55.0 | |
| 0.50% NU-100 | 152.1 | 167.5 | 71.8 | 55.0 | |
| 0.05% SF-B01 | - | 164.0 | 0 | 52.5 | |
| 0.05% SF-B01 | 151.1 | 167.7 | 78.6 | 49.6 | |
| 0.10% SF-B01 | 151.7 | 167.5 | 78.6 | 50.2 | |
| 0.25% SF-B01 | 151.9 | 167.4 | 78.9 | 50.8 | |
| 0.50% SF-B01 | 152.2 | 167.5 | 78.8 | 51.9 | |
| Injection molded, 3 mm | iPP | - | 163.3 | 0 | 40.8 |
| 0.01% NU-100 | - | 163.9 | 0 | 52.9 | |
| 0.05% NU-100 | 151.7 | 167.6 | 77.2 | 50.3 | |
| 0.10% NU-100 | 152.0 | 167.6 | 79.3 | 49.9 | |
| 0.25% NU-100 | 152.3 | 167.6 | 79.4 | 50.4 | |
| 0.50% NU-100 | 151.6 | 167.0 | 79.3 | 55.4 | |
| 0.01% SF-B01 | - | 163.4 | 0 | 48.1 | |
| 0.05% SF-B01 | 151.5 | 167.4 | 78.0 | 51.0 | |
| 0.10% SF-B01 | 151.6 | 167.9 | 81.0 | 47.9 | |
| 0.25% SF-B01 | 152.2 | 167.5 | 78.2 | 50.83 | |
| 0.50% SF-B01 | 152.1 | 167.4 | 78.4 | 49.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barczewski, M.; Mysiukiewicz, O.; Dutkiewicz, M.; Szołyga, M.; Dobrzyńska-Mizera, M.; Piasecki, A. Effect of Shear Stress during Processing on Structure, Morphology, and Properties of Isotactic Polypropylene Nucleated with Silsesquioxane-Based β-Nucleating Agent. Materials 2023, 16, 3627. https://doi.org/10.3390/ma16103627
Barczewski M, Mysiukiewicz O, Dutkiewicz M, Szołyga M, Dobrzyńska-Mizera M, Piasecki A. Effect of Shear Stress during Processing on Structure, Morphology, and Properties of Isotactic Polypropylene Nucleated with Silsesquioxane-Based β-Nucleating Agent. Materials. 2023; 16(10):3627. https://doi.org/10.3390/ma16103627
Chicago/Turabian StyleBarczewski, Mateusz, Olga Mysiukiewicz, Michał Dutkiewicz, Mariusz Szołyga, Monika Dobrzyńska-Mizera, and Adam Piasecki. 2023. "Effect of Shear Stress during Processing on Structure, Morphology, and Properties of Isotactic Polypropylene Nucleated with Silsesquioxane-Based β-Nucleating Agent" Materials 16, no. 10: 3627. https://doi.org/10.3390/ma16103627
APA StyleBarczewski, M., Mysiukiewicz, O., Dutkiewicz, M., Szołyga, M., Dobrzyńska-Mizera, M., & Piasecki, A. (2023). Effect of Shear Stress during Processing on Structure, Morphology, and Properties of Isotactic Polypropylene Nucleated with Silsesquioxane-Based β-Nucleating Agent. Materials, 16(10), 3627. https://doi.org/10.3390/ma16103627