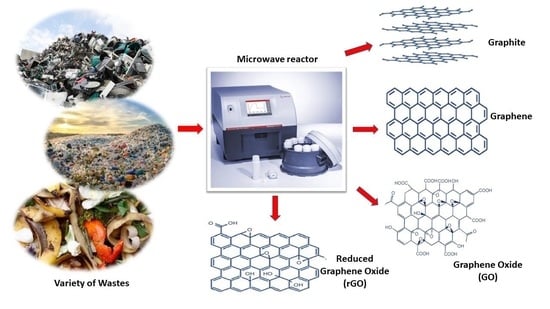
An Overview of Recycling Wastes into Graphene Derivatives Using Microwave Synthesis; Trends and Prospects
Abstract
:1. Introduction
2. Synthesis of Waste into Graphene Derivatives
2.1. Biowastes
2.2. Coal Waste
2.3. Industrial Wastes
3. Microwave Synthesis of Graphene Nanomaterials from Waste Materials
4. Characterization Techniques
4.1. X-ray Diffraction (XRD) and X-ray Photoelectron Spectroscopy (XPS)
4.2. Other Characterization Methods
4.2.1. Raman Spectroscopy and Fourier-Transform Infrared Spectroscopy (FTIR)
4.2.2. Atomic Force Microscopy (AFM)
4.2.3. Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDS)
4.2.4. Transmission Electron Microscopy (TEM) and High-Resolution Transmission Electron Microscopy (HRTEM)
4.2.5. Field Emission Scanning Electron Microscopy (FESEM)
5. Future Prospects
- Optimization of process variables and techniques to regulate the size, quality, and morphology of graphene-derived materials from waste materials.
- Improved synthetic concepts and methods are highly inspiring and necessitate commercial research involving renewable and biodegradable waste materials.
- Well-ordered oxidation/decrease and functionalization are expected for calibrating material properties, for example, band hole, electrical conductivity, and mechanical properties [170].
- Controlled graphite, GO, and rGO adjustment is in this way basic for widening the utilizations of graphene-based materials.
- To survey the wellbeing risk related with graphene and its subsidiaries, the poisonousness and biocompatibility of these unique carbon structures and their subordinates should be examined [171].
- Due to its extensive property, graphene preparation is a crucial area for material scientists. As a result, the scientific community should focus on advanced and novel microwave instruments which would be a great substitute of toxic and harsh chemicals
- To explore more variations that involving novel synthetic techniques, high purity GO for its mass production.
- There should be more consideration to lessen the cost effects of graphene derivatives.
- There should be more emphasis on the high yield and purity of graphene derivatives using a variety of wastes through microwave synthesis.
- This may also lead towards the excellence of functionalization, such as ID, 2D, and 3D graphene members, to fabricate waste materials into graphene-based structures with enhanced functionalities and high surface areas [172].
- Improving synthetic ideas and microwave approaches are remarkably motivating and requires further investigations by recycling waste materials for the optimization of parameters, such as time, power, and frequency.
- Further analysis of microwave synthesis and applications should be explored where the waste-based graphene derivatives can be utilized and, thus, the structures and properties can be modified as per the industrial demands.

6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| MSW | Metropolitan strong squanders |
| GSs | Graphene sheets |
| MPCVD | Microwave plasma assisted chemical vapor deposition |
| PIL-rGO | Poly ionic liquid reduced graphene oxide |
| ErGO | Electrochemically reduced graphene oxide |
| DBD | Dielectric barrier discharge |
| TX-NC-GO and TX-C-GO | Coal-based graphite-like carbons oxides |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| TEM | Transmission electron microscopy |
| FESEM | Field emission scanning electron microscopy |
| ANOVA | Analysis of variance |
| BBD Design | Box-behnken design |
| SEMEDAX | Scanning electron microscopy energy dispersive X-Ray |
| EDS | Energy dispersive spectroscopy |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| AFM | Atomic force microscopy |
| SAED | Selected area (electron) diffraction |
| FETEM | Field-emission transmission electron microscope |
References
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes–A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Luo, B.; Liu, S.; Zhi, L. Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 2012, 8, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, X.; Hu, J.; Xu, H. Low threshold optical bistability based on coupled graphene Tamm states. Results Phys. 2021, 21, 103824. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, F.; Bie, X.; Ou, P.; Song, J. Atomistic and continuum modeling of 3D graphene honeycombs under uniaxial in-plane compression. Comput. Mater. Sci. 2021, 197, 110646. [Google Scholar] [CrossRef]
- Safian, M.T.U.; Umar, K.; Ibrahim, M.N.M. Synthesis and scalability of graphene and its derivatives: A journey towards sustainable and commercial material. J. Clean. Prod. 2021, 318, 128603. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Somanathan, T.; Prasad, K.; Ostrikov, K.K.; Saravanan, A.; Krishna, V.M. Graphene oxide synthesis from agro waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Tan, P.H.; Han, W.P.; Zhao, W.J.; Wu, Z.H.; Chang, K.; Wang, H.; Wang, Y.F.; Bonini, N.; Marzari, N.; Pugno, N.; et al. The shear mode of multilayer graphene. Nat. Mater. 2012, 11, 294–300. [Google Scholar] [CrossRef]
- Vasu, K.S.; Prestat, E.; Abraham, J.; Dix, J.; Kashtiban, R.J.; Beheshtian, J.; Sloan, J.; Carbone, P.; Neek-Amal, M.; Haigh, S.J.; et al. Van der Waals pressure and its effect on trapped interlayer molecules. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Dasari, B.L.; Nouri, J.M.; Brabazon, D.; Naher, S. Graphene and derivatives–Synthesis techniques, properties and their energy applications. Energy 2017, 140, 766–778. [Google Scholar] [CrossRef]
- Junginger, H.M.; Jonker, J.G.G.; Faaij, A.P.C.; Cocchi, M.; Schouwenberg, P. Summary, Synthesis and Conclusions from IEA Bioenergy Task 40 Country Reports on International Bioenergy Trade; Utrecht University: Utrecht, The Netherlands, 2011. [Google Scholar]
- Lehr, J.H.; Keeley, J. (Eds.) Alternative Energy and Shale Gas Encyclopedia; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hakeem, K.R.; Jawaid, M.; Alothman, O.Y. (Eds.) Agricultural Biomass Based Potential Materials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Ahmad, W. Advances in synthesis of graphene derivatives using industrial wastes precursors; prospects and challenges. J. Mater. Res. Technol. 2020, 9, 15924–15951. [Google Scholar] [CrossRef]
- Xie, X.; Goodell, B. Thermal degradation and conversion of plant biomass into high value carbon products. In Deterioration and Protection of Sustainable Biomaterials; American Chemical Society: Washington, DC, USA, 2014; pp. 147–158. [Google Scholar]
- He, L.; Guo, S.; Lei, J.; Sha, Z.; Liu, Z. The effect of Stone–Thrower–Wales defects on mechanical properties of graphene sheets—A molecular dynamics study. Carbon 2014, 75, 124–132. [Google Scholar] [CrossRef]
- Ronsse, F.; Nachenius, R.W.; Prins, W. Carbonization of biomass. In Recent Advances in Thermo-Chemical Conversion of Biomass; Elsevier: Amsterdam, The Netherlands, 2015; pp. 293–324. [Google Scholar]
- Zhang, C.; Guimin, L.U.; Ze, S.U.N.; Jianguo, Y.U. Catalytic graphitization of carbon/carbon composites by lanthanum oxide. J. Rare Earths 2012, 30, 128–132. [Google Scholar] [CrossRef]
- Pan, G.; Liang, W.; Liang, P.; Chen, Q. Effect of vacuum-carbonization treatment of soft carbon anodes derived from coal-based mesophase pitch for lithium-ion batteries. Clean Energy 2019, 3, 211–216. [Google Scholar] [CrossRef]
- Ikram, R.; Mohamed Jan, B.; Nagy, P.B.; Szabo, T. Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective. Nanotechnol. Rev. 2023, 12, 20220512. [Google Scholar] [CrossRef]
- Safian, M.T.U.; Haron, U.S.; Ibrahim, M.M. A review on bio-based graphene derived from biomass wastes. BioResources 2020, 15, 9756. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X. Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J. Electroanal. Chem. 2016, 768, 18–26. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Singh, R.K.; Singh, D.P.; Moshkalev, S.A. Recent advances in the synthesis and modification of carbon-based 2D materials for application in energy conversion and storage. Prog. Energy Combust. Sci. 2018, 67, 115–157. [Google Scholar] [CrossRef]
- Ray, A.K.; Sahu, R.K.; Rajinikanth, V.; Bapari, H.; Ghosh, M.; Paul, P. Preparation and characterization of graphene and Ni-decorated graphene using flower petals as the precursor material. Carbon 2012, 50, 4123–4129. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Shams, S.S.; Zhang, L.S.; Hu, R.; Zhang, R.; Zhu, J. Synthesis of graphene from biomass: A green chemistry approach. Mater. Lett. 2015, 161, 476–479. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Yusof, N.A.; Zainal, Z. Oil palm waste-based precursors as a renewable and economical carbon sources for the preparation of reduced graphene oxide from graphene oxide. Nanomaterials 2017, 7, 182. [Google Scholar] [CrossRef]
- Tahir, N.A.M.; Abdollah, M.F.B.; Tamaldin, N.; Amiruddin, H.; Tokoroyama, T.; Umehara, N. Potential of growing graphene from solid waste products. In Proceedings of the SAKURA Symposium on Mechanical Science and Engineering, Nagoya, Japan, 12 September 2017; pp. 26–28. [Google Scholar]
- Ismail, M.S.; Yusof, N.; Yusop, M.Z.M.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Karim, Z.A. Synthesis and characterization of graphene derived from rice husks. Malays. J. Fundam. Appl. Sci. 2019, 15, 516–521. [Google Scholar] [CrossRef]
- Mamat, R.H.; Hamzah, F.; Hashim, A.; Abdullah, S.; Alrokayan, S.A.; Khan, H.A.; Safiay, M.; Jafar, S.M.; Asli, A.; Khusaimi, Z.; et al. Influence of volume variety of waste cooking palm oil as carbon source on graphene growth through double thermal chemical vapor deposition. In Proceedings of the 2018 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 15–17 August 2018; pp. 53–56. [Google Scholar]
- Sun, Z.; Zheng, M.; Hu, H.; Dong, H.; Liang, Y.; Xiao, Y.; Lei, B.; Liu, Y. From biomass wastes to vertically aligned graphene nanosheet arrays: A catalyst-free synthetic strategy towards high-quality graphene for electrochemical energy storage. Chem. Eng. J. 2018, 336, 550–561. [Google Scholar] [CrossRef]
- Shah, J.; Lopez-Mercado, J.; Carreon, M.G.; Lopez-Miranda, A.; Carreon, M.L. Plasma synthesis of graphene from mango peel. ACS Omega 2018, 3, 455–463. [Google Scholar] [CrossRef]
- Lu, X.; Xiang, K.; Zhou, W.; Zhu, Y.; He, Y.; Chen, H. Graphene-like carbon derived from macadamia nut shells for high-performance supercapacitor. Russ. J. Electrochem. 2019, 55, 242–246. [Google Scholar] [CrossRef]
- Sha, T.; Liu, J.; Sun, M.; Li, L.; Bai, J.; Hu, Z.; Zhou, M. Green and low-cost synthesis of nitrogen-doped graphene-like mesoporous nanosheets from the biomass waste of okara for the amperometric detection of vitamin C in real samples. Talanta 2019, 200, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Widiatmoko, P.; Sukmana, I.F.; Nurdin, I.; Prakoso, T.; Devianto, H. Increasing yield of graphene synthesis from oil palm empty fruit bunch via two-stages pyrolysis. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 543, p. 012032. [Google Scholar]
- Gupta, K.; Gupta, D.; Khatri, O.P. Applied Surface Science Graphene-like porous carbon nanostructure from Bengal gram bean husk and its application for fast and efficient adsorption of organic dyes. Appl. Surf. Sci. 2019, 476, 647–657. [Google Scholar] [CrossRef]
- Ekhlasi, L.; Younesi, H.; Rashidi, A.; Bahramifar, N. Populus wood biomass-derived graphene for high CO2 capture at atmospheric pressure and estimated cost of production. Process Saf. Environ. Prot. 2018, 113, 97–108. [Google Scholar] [CrossRef]
- Ding, Z.; Yuan, T.; Wen, J.; Cao, X.; Sun, S.; Xiao, L.P.; Shi, Q.; Wang, X.; Sun, R. Green synthesis of chemical converted graphene sheets derived from pulping black liquor. Carbon 2020, 158, 690–697. [Google Scholar] [CrossRef]
- Roquia, A.; khalfan hamed Alhashmi, A.; hamed Abdullah alhasmi, B. Synthesis and characterisation of carbon nanotubes from waste of Juglans regia (walnut) shells. Fuller. Nanotub. Carbon Nanostructures 2021, 29, 860–867. [Google Scholar] [CrossRef]
- Ramli, R.; Hidayat, R. Graphene Oxide Based on Biomass Waste: Synthesis and Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mahajan, R.L. A facile method for coal to graphene oxide and its application to a biosensor. Carbon 2021, 181, 408–420. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Zhang, G.; Guo, Y.; Zhang, J.; Li, G. Pyrolysis characteristics and kinetics of two Chinese low-rank coals. J. Therm. Anal. Calorim. 2015, 122, 975–984. [Google Scholar] [CrossRef]
- Xu, T.; Srivatsa, S.C.; Bhattacharya, S. In-situ synchrotron IR study on surface functional group evolution of Victorian and Thailand low-rank coals during pyrolysis. J. Anal. Appl. Pyrolysis 2016, 122, 122–130. [Google Scholar] [CrossRef]
- Zhu, Z.; Zuo, H.; Li, S.; Tu, J.; Guan, W.; Song, W.L.; Zhao, J.; Tian, D.; Jiao, S. A green electrochemical transformation of inferior coals to crystalline graphite for stable Li-ion storage. J. Mater. Chem. A 2019, 7, 7533–7540. [Google Scholar] [CrossRef]
- Kaklidis, N.; Kyriakou, V.; Marnellos, G.E.; Strandbakke, R.; Arenillas, A.; Menéndez, J.A.; Konsolakis, Μ. Effect of fuel thermal pretreament on the electrochemical performance of a direct lignite coal fuel cell. Solid State Ion. 2016, 288, 140–146. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, B.; Fan, X.; Liang, P.; Zhao, G.; Saikia, B.K.; Wei, X. Hierarchical porous carbon derived from coal and biomass for high performance supercapacitors. Fuel 2022, 311, 122552. [Google Scholar] [CrossRef]
- Li, K.K.; Liu, G.Y.; Zheng, L.S.; Jia, J.; Zhu, Y.Y.; Zhang, Y.T. Coal-derived carbon nanomaterials for sustainable energy storage applications. New Carbon Mater. 2021, 36, 133–154. [Google Scholar] [CrossRef]
- Savitskii, D.P. Preparation and characterization of colloidal dispersions of graphene-like structures from different ranks of coals. J. Fuel Chem. Technol. 2017, 45, 897–907. [Google Scholar] [CrossRef]
- Pakhira, B.; Ghosh, S.; Maity, S.; Sangeetha, D.N.; Laha, A.; Allam, A.; Sarkar, S. Extraction of preformed graphene oxide from coal: Its clenched fist form entrapping large molecules. RSC Adv. 2015, 5, 89076–89082. [Google Scholar] [CrossRef]
- Tran, V.T.; Saint-Martin, J.; Dollfus, P. Electron transport properties of graphene nanoribbons with Gaussian deformation. Phys. Rev. B 2020, 102, 075425. [Google Scholar] [CrossRef]
- Canel, M.; Mısırlıoğlu, Z.; Canel, E.; Bozkurt, P.A. Distribution and comparing of volatile products during slow pyrolysis and hydropyrolysis of Turkish lignites. Fuel 2016, 186, 504–517. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Z.; Zhang, Y.; Meng, B.; Zhou, A.; Qiu, J. Graphene sheets from graphitized anthracite coal: Preparation, decoration, and application. Energy Fuels 2012, 26, 5186–5192. [Google Scholar] [CrossRef]
- Abidi, I.H.; Liu, Y.; Pan, J.; Tyagi, A.; Zhuang, M.; Zhang, Q.; Cagang, A.A.; Weng, L.T.; Sheng, P.; Goddard, W.A., III; et al. Regulating Top-Surface Multilayer/Single-Crystal Graphene Growth by “Gettering” Carbon Diffusion at Backside of the Copper Foil. Adv. Funct. Mater. 2017, 27, 1700121. [Google Scholar] [CrossRef]
- Jadhav, U.U.; Hocheng, H. A review of recovery of metals from industrial waste. J. Achiev. Mater. Manuf. Eng. 2012, 54, 159–167. [Google Scholar]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives–A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Simandl, G.J.; Paradis, S.; Akam, C. Graphite deposit types, their origin, and economic significance. Br. Columbia Minist. Energy Mines Br. Columbia Geol. Surv. 2015, 3, 163–171. [Google Scholar]
- Zhang, Z.J.; Simionesie, D.; Schaschke, C. Graphite and hybrid nanomaterials as lubricant additives. Lubricants 2014, 2, 44–65. [Google Scholar] [CrossRef]
- Xuan, Y.; Jiang, G.; Li, Y. Nanographite oxide as ultrastrong fluid-loss-control additive in water-based drilling fluids. J. Dispers. Sci. Technol. 2014, 35, 1386–1392. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Siaw, W.C.; Tsuji, T.; Manaf, N.A.; Patah, M.A.; Jan, B.M. Synthesis of graphene oxide from industrial waste. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 778, p. 012050. [Google Scholar]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Nakamura, N.; Reeja-Jayan, B. Synchrotron X-ray characterization of materials synthesized under microwave irradiation. J. Mater. Res. 2019, 34, 194–205. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.; Chao, D.; Guan, C.; Zhang, Y.; Li, L.; Ge, X.; Bacho, I.M.; Tu, J.; Fan, H.J. Solution synthesis of metal oxides for electrochemical energy storage applications. Nanoscale 2014, 6, 5008–5048. [Google Scholar] [CrossRef]
- Wong, C.H.A.; Jankovský, O.; Sofer, Z.; Pumera, M. Vacuum-assisted microwave reduction/exfoliation of graphite oxide and the influence of precursor graphite oxide. Carbon 2014, 77, 508–517. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Graser, J.; Zhao, R.; Gao, F.; O’Connell, M.J. Solution-based carbohydrate synthesis of individual solid, hollow, and porous carbon nanospheres using spray pyrolysis. ACS Nano 2013, 7, 11156–11165. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Ikram, R.; Mohamed Jan, B.; Abdul Qadir, M.; Sidek, A.; Stylianakis, M.M.; Kenanakis, G. Recent advances in chitin and chitosan/graphene-based bio-nanocomposites for energetic applications. Polymers 2021, 13, 3266. [Google Scholar] [CrossRef]
- Tatarova, E.; Dias, A.; Henriques, J.; do Rego, A.B.; Ferraria, A.M.; Abrashev, M.V.; Luhrs, C.C.; Phillips, J.; Dias, F.M.; Ferreira, C.M. Microwave plasmas applied for the synthesis of free standing graphene sheets. J. Phys. D Appl. Phys. 2014, 47, 385501. [Google Scholar] [CrossRef]
- Zheng, Y.; Lv, K.; Wang, Z.; Deng, K.; Li, M. Microwave-assisted rapid synthesis of anatase TiO2 nanocrystals with exposed {0 0 1} facets. J. Mol. Catal. A Chem. 2012, 356, 137–143. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J. Novel template-assisted microwave conversion of graphene oxide to graphene patterns: A reduction transfer mechanism. Carbon 2019, 148, 159–163. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk biomass: A comprehensive utilization strategy. ACS Appl. Mater. Interfaces 2016, 8, 1434–1439. [Google Scholar] [CrossRef]
- Van Khai, T.; Kwak, D.S.; Kwon, Y.J.; Cho, H.Y.; Huan, T.N.; Chung, H.; Ham, H.; Lee, C.; Van Dan, N.; Tung, N.T.; et al. Direct production of highly conductive graphene with a low oxygen content by a microwave-assisted solvothermal method. Chem. Eng. J. 2013, 232, 346–355. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, Q.; Wang, J. A simple and fast microwave assisted approach for the reduction of graphene oxide. Ceram. Int. 2016, 42, 3007–3013. [Google Scholar] [CrossRef]
- Sengupta, J. Different synthesis routes of graphene-based metal nanocomposites. arXiv 2019, arXiv:1911.01720. [Google Scholar]
- Thomas, R.; Rao, K.Y.; Rao, G.M. Morphology and electrochemical performance of graphene nanosheet array for Li-ion thin film battery. Electrochim. Acta 2013, 108, 458–464. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, J.G.; Jie, X.; Liu, W.; Liu, F.; Yin, Y.; Gu, J.; Zou, Z. Preparation and electrochemical characterization of nitrogen doped graphene by microwave as supporting materials for fuel cell catalysts. Electrochim. Acta 2012, 60, 354–358. [Google Scholar] [CrossRef]
- Adolfsson, K.H.; Hassanzadeh, S.; Hakkarainen, M. Valorization of cellulose and waste paper to graphene oxide quantum dots. RSC Adv. 2015, 5, 26550–26558. [Google Scholar] [CrossRef]
- Agarkar, S.; Yadav, P.; Fernandes, R.; Kothari, D.; Suryawanshi, A.; Ogale, S. Minute-made activated porous carbon from agro-waste for Li-ion battery anode using a low power microwave oven. Electrochim. Acta 2016, 212, 535–544. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, J.; Vaidhyanathan, B.; Zhang, H.; Anshuman, A.; Nare, A.; Saremi-Yarahmadi, S. Rapid microwave-assisted bulk production of high-quality reduced graphene oxide for lithium ion batteries. Materialia 2020, 13, 100833. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. J. Saudi Soc. Agric. Sci. 2017, 16, 41–48. [Google Scholar] [CrossRef]
- 88. Abbas, A.; Tabish, T.A.; Bull, S.J.; Lim, T.M.; Phan, A.N. High yield synthesis of graphene quantum dots from biomass waste as a highly selective probe for Fe3+ sensing. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Liu, C.; Chen, W.; Hong, S.; Pan, M.; Jiang, M.; Wu, Q.; Mei, C. Fast microwave synthesis of hierarchical porous carbons from waste palm boosted by activated carbons for supercapacitors. Nanomaterials 2019, 9, 405. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Transforming waste poly (ethylene terephthalate) into nitrogen doped carbon nanotubes and its utility in oxygen reduction reaction and bisphenol-a removal from contaminated water. Materials 2020, 13, 4144. [Google Scholar] [CrossRef]
- Yanti, D.R.; Hikmah, U.; Prasetyo, A.; Hastuti, E. The effect of microwave irradiation on reduced graphene oxide from coconut shells. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 456, p. 012008. [Google Scholar]
- Fu, M.; Zhu, Z.; Zhang, Z.; Zhuang, Q.; Chen, W.; Liu, Q. Microwave deposition synthesis of Ni (OH) 2/sorghum stalk biomass carbon electrode materials for supercapacitors. J. Alloy. Compd. 2020, 846, 156376. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Ramesh, M.; Lekshmi, G.S.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Graphene oxide–Based supercapacitors from agricultural wastes: A step to mass production of highly efficient electrodes for electrical transportation systems. Renew. Energy 2020, 151, 731–739. [Google Scholar] [CrossRef]
- Chalmpes, N.; Asimakopoulos, G.; Baikousi, M.; Moschovas, D.; Avgeropoulos, A.; Bourlinos, A.B.; Sedajova, V.; Bakandritsos, A.; Gournis, D.; Karakassides, M.A. Fast and direct microwave synthesis of carbon from bovine blood waste: A feedstock material for extractive metallurgy, carbon dots production and graphite synthesis. J. Nanotechnol. Res. 2021, 3, 011–028. [Google Scholar]
- Parveen, R.A.; Rakkesh, R.A.; Durgalakshmi, D.; Balakumar, S. Graphene-Ag2S hybrid nanostructures: A hybrid gas sensor for room temperature hydrogen sensing application. Mater. Lett. 2021, 303, 130470. [Google Scholar] [CrossRef]
- Ersan, M.; Dogan, H. Development of new adsorbents via microwave treatment magnetic PET synthesis from waste PET and investigation of TC removal. Colloid Interface Sci. Commun. 2021, 42, 100416. [Google Scholar] [CrossRef]
- Irez, A.B.; Okan, C.; Kaya, R.; Cebe, E. Development of recycled disposable mask based polypropylene matrix composites: Microwave self-healing via graphene nanoplatelets. Sustain. Mater. Technol. 2022, 31, e00389. [Google Scholar] [CrossRef]
- Danial, W.H.; Abdullah, M.; Bakar, M.A.A.; Yunos, M.S.; Ibrahim, A.R.; Iqbal, A.; Adnan, N.N. The valorisation of grass waste for the green synthesis of graphene quantum dots for nonlinear optical applications. Opt. Mater. 2022, 132, 112853. [Google Scholar] [CrossRef]
- Kumar, D.; Rani, S.; Nandan, B.; Srivastava, R.K. Nonpolar Graphene Quantum Dot-Based Hydrophobic Coating from Microwave-Assisted Treatment of Styrofoam Waste. ACS Sustain. Chem. Eng. 2022, 10, 1070–1077. [Google Scholar] [CrossRef]
- Wang, Y.; Srinivasakannan, C.; Wang, H.; Xue, G.; Wang, L.; Wang, X.; Duan, X. Preparation of novel biochar containing graphene from waste bamboo with high methylene blue adsorption capacity. Diam. Relat. Mater. 2022, 125, 109034. [Google Scholar] [CrossRef]
- Khose, R.V.; Bangde, P.; Bondarde, M.P.; Dhumal, P.S.; Bhakare, M.A.; Chakraborty, G.; Ray, A.K.; Dandekar, P.; Some, S. Waste derived approach towards wealthy fluorescent N-doped graphene quantum dots for cell imaging and H2O2 sensing applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120453. [Google Scholar] [CrossRef]
- Hong, W.T.; Moon, B.K.; Yang, H.K. Microwave irradiation and color converting film application of carbon dots originated from wasted toner powder. Mater. Res. Bull. 2022, 156, 111999. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Zhang, R.; Thiyagarajan, P.; Tiede, D.M. Probing protein fine structures by wide angle solution X-ray scattering. J. Appl. Crystallogr. 2020, 33, 565–568. [Google Scholar] [CrossRef]
- Spyrou, K.; Rudolf, P. An introduction to graphene. In Functionalization of Graphene; Wiley: Hoboken, NJ, USA, 2014; pp. 1–20. [Google Scholar]
- Yousefi, N.; Lu, X.; Elimelech, M.; Tufenkji, N. Environmental performance of graphene-based 3D macrostructures. Nat. Nanotechnol. 2019, 14, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tang, Y.; Xi, J.; Kong, J. Functionalized graphene sheets with poly (ionic liquid) s and high adsorption capacity of anionic dyes. Appl. Surf. Sci. 2015, 326, 276–284. [Google Scholar] [CrossRef]
- Mu, S.J.; Su, Y.C.; Xiao, L.H.; Liu, S.D.; Hu, T.; Tang, H.B. X-ray difraction pattern of graphite oxide. Chin. Phys. Lett. 2013, 30, 096101. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Y.; Wu, F.; Yang, C.; Zeng, F.; Wu, Q. Graphene oxide: The mechanisms of oxidation and exfoliation. J. Mater. Sci. 2012, 47, 4400–4409. [Google Scholar] [CrossRef]
- Buchner, F.; Forster-Tonigold, K.; Bozorgchenani, M.; Gross, A.; Behm, R.J. Interaction of a self-assembled ionic liquid layer with graphite (0001): A combined experimental and theoretical study. J. Phys. Chem. Lett. 2016, 7, 226–233. [Google Scholar] [CrossRef]
- Zaldivar, R.J.; Adams, P.M.; Nokes, J.; Kim, H.I. Surface functionalization of graphene like materials by carbon monoxide atmospheric plasma treatment for improved wetting without structural degradation. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2012, 30, 03D107. [Google Scholar]
- Amadei, C.A.; Arribas, P.; Vecitis, C.D. Graphene oxide standardization and classification: Methods to support the leap from lab to industry. Carbon 2018, 133, 398–409. [Google Scholar] [CrossRef]
- Rosenzweig, S.; Sorial, G.A.; Sahle-Demessie, E.; McAvoy, D.C. Optimizing the physical-chemical properties of carbon nanotubes (CNT) and graphene nanoplatelets (GNP) on Cu (II) adsorption. J. Hazard. Mater. 2014, 279, 410–417. [Google Scholar] [CrossRef]
- Maccaferri, G.; Zanardi, C.; Xia, Z.Y.; Kovtun, A.; Liscio, A.; Terzi, F.; Palermo, V.; Seeber, R. Systematic study of the correlation between surface chemistry, conductivity and electrocatalytic properties of graphene oxide nanosheets. Carbon 2017, 120, 165–175. [Google Scholar] [CrossRef]
- Shin, Y.E.; Sa, Y.J.; Park, S.; Lee, J.; Shin, K.H.; Joo, S.H.; Ko, H. An ice-templated, pH-tunable self-assembly route to hierarchically porous graphene nanoscroll networks. Nanoscale 2014, 6, 9734–9741. [Google Scholar] [CrossRef]
- Huang, H.H.; De Silva, K.K.H.; Kumara, G.R.A.; Yoshimura, M. Structural evolution of hydrothermally derived reduced graphene oxide. Sci. Rep. 2018, 8, 6849. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, H.; Zhou, W.; Li, G.; Liang, X.; Guo, J.; Tang, S. Low temperature reduction of graphene oxide film by ammonia solution and its application for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 10098–10105. [Google Scholar] [CrossRef]
- Muralikrishna, S.; Sureshkumar, K.; Varley, T.S.; Nagaraju, D.H.; Ramakrishnappa, T. In situ reduction and functionalization of graphene oxide with L-cysteine for simultaneous electrochemical determination of cadmium (II), lead (II), copper (II), and mercury (II) ions. Anal. Methods 2014, 6, 8698–8705. [Google Scholar] [CrossRef]
- Feng, J.; Hou, Y.; Wang, X.; Quan, W.; Zhang, J.; Wang, Y.; Li, L. In-depth study on adsorption and photocatalytic performance of novel reduced graphene oxide-ZnFe22O4-polyaniline composites. J. Alloy. Compd. 2016, 681, 157–166. [Google Scholar] [CrossRef]
- Khan, A.J.; Khan, A.; Javed, M.S.; Arshad, M.; Asim, S.; Khalid, M.; Siyal, S.H.; Hussain, S.; Hanif, M.; Liu, Z. Surface assembly of Fe3O4 nanodiscs embedded in reduced graphene oxide as a high-performance negative electrode for supercapacitors. Ceram. Int. 2020, 46, 19499–19505. [Google Scholar] [CrossRef]
- Xing, T.; Zheng, Y.; Li, L.H.; Cowie, B.C.; Gunzelmann, D.; Qiao, S.Z.; Huang, S.; Chen, Y. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene. Acs Nano 2014, 8, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Ikram, R.; Mohamed Jan, B.; Atif Pervez, S.; Papadakis, V.M.; Ahmad, W.; Bushra, R.; Kenanakis, G.; Rana, M. Recent advancements of N-doped graphene for rechargeable batteries: A review. Crystals 2020, 10, 1080. [Google Scholar] [CrossRef]
- Cao, X.; Hong, T.; Yang, R.; Tian, J.H.; Xia, C.; Dong, J.C.; Li, J.F. Insights into the catalytic activity of barium carbonate for oxygen reduction reaction. J. Phys. Chem. C 2016, 120, 22895–22902. [Google Scholar] [CrossRef]
- Whelan, P.R.; Jessen, B.S.; Wang, R.; Luo, B.; Stoot, A.C.; Mackenzie, D.M.; Braeuninger-Weimer, P.; Jouvray, A.; Prager, L.; Camilli, L.; et al. Raman spectral indicators of catalyst decoupling for transfer of CVD grown 2D materials. Carbon 2017, 117, 75–81. [Google Scholar] [CrossRef]
- Barbon, A.; Tampieri, F. Identification of slow relaxing spin components by pulse EPR techniques in graphene-related materials. AIMS Mater Sci. 2017, 4, 147–157. [Google Scholar] [CrossRef]
- Qi, B.; Ren, K.; Lin, Y.; Zhang, S.; Wei, T.; Fan, Z. Design of layered-stacking graphene assemblies as advanced electrodes for supercapacitors. Particuology 2022, 60, 1–13. [Google Scholar] [CrossRef]
- Kumar, T.N.; Vardhan, K.V.; Krishna, M.H.; Nagaraja, P.V. Effect of graphene oxide on strength properties of cementitious materials: A review. Mater. Today Proc. 2021, 46, 2157–2160. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Z.; Yang, L.; Shi, H.; Fang, D.; Wang, M.; Shao, P.; Luo, X. Electrochemical approach toward reduced graphene oxide-based electrodes for environmental applications: A review. Sci. Total Environ. 2021, 778, 146301. [Google Scholar] [CrossRef]
- Goodwin, S.; Coldrick, Z.; Heeg, S.; Grieve, B.; Vijayaraghavan, A.; Hill, E.W. Fabrication and electrochemical response of pristine graphene ultramicroelectrodes. Carbon 2021, 177, 207–215. [Google Scholar] [CrossRef]
- He, D.; Wang, W.; Fu, Y.; Zhao, R.; Xue, W.; Hu, W. Formation of three-dimensional honeycomb-like nitrogen-doped graphene for use in energy-storage devices. Compos. Part A Appl. Sci. Manuf. 2016, 91, 140–144. [Google Scholar] [CrossRef]
- Hu, M.; Wang, X. Effect of N3− species on selective acetylene hydrogenation over Pd/SAC catalysts. Catal. Today 2016, 263, 98–104. [Google Scholar] [CrossRef]
- Lin, C.; Wei, W.; Hu, Y.H. Catalytic behavior of graphene oxide for cement hydration process. J. Phys. Chem. Solids 2016, 89, 128–133. [Google Scholar] [CrossRef]
- Yao, H.; Li, F.; Lutkenhaus, J.; Kotaki, M.; Sue, H.J. High-performance photocatalyst based on nanosized ZnO-reduced graphene oxide hybrid for removal of Rhodamine B under visible light irradiation. AIMS Mater Sci. 2016, 3, 1410–1425. [Google Scholar]
- Geng, X.; Guo, Y.; Li, D.; Li, W.; Zhu, C.; Wei, X.; Chen, M.; Gao, S.; Qiu, S.; Gong, Y.; et al. Interlayer catalytic exfoliation realizing scalable production of large-size pristine few-layer graphene. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Liu, Y.; Wang, W.; Wynn, J.; Gao, J. Using glucosamine as a reductant to prepare reduced graphene oxide and its nanocomposites with metal nanoparticles. J. Nanoparticle Res. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Chua, C.K.; Ambrosi, A.; Pumera, M. Graphene oxide reduction by standard industrial reducing agent: Thiourea dioxide. J. Mater. Chem. 2012, 22, 11054–11061. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, H.; Lu, J.; Fu, Q.; Chu, Y. Graphene nanosheets synthesis via chemical reduction of graphene oxide using sodium acetate trihydrate solution. Synth. Met. 2014, 193, 132–138. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Han, H.S.; Jeon, S. One-step chemical reduction of graphene oxide with oligothiophene for improved electrocatalytic oxygen reduction reactions. Carbon 2013, 61, 164–172. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef]
- Chen, H.; Du, W.; Liu, J.; Qu, L.; Li, C. Efficient room-temperature production of high-quality graphene by introducing removable oxygen functional groups to the precursor. Chem. Sci. 2019, 10, 1244–1253. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapour deposition of graphene—Synthesis, characterisation, and applications: A review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef] [PubMed]
- Li, N.W.; Shi, Y.; Yin, Y.X.; Zeng, X.X.; Li, J.Y.; Li, C.J.; Wan, L.J.; Wen, R.; Guo, Y.G. A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. 2018, 130, 1521–1525. [Google Scholar] [CrossRef]
- Baldassarre, L.; Giliberti, V.; Rosa, A.; Ortolani, M.; Bonamore, A.; Baiocco, P.; Kjoller, K.; Calvani, P.; Nucara, A. Mapping the amide I absorption in single bacteria and mammalian cells with resonant infrared nanospectroscopy. Nanotechnology 2016, 27, 075101. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Pyne, A.L.; Noy, A.; Main, K.H.; Velasco-Berrelleza, V.; Piperakis, M.M.; Mitchenall, L.A.; Cugliandolo, F.M.; Beton, J.G.; Stevenson, C.E.; Hoogenboom, B.W.; et al. Base-pair resolution analysis of the effect of supercoiling on DNA flexibility and major groove recognition by triplex-forming oligonucleotides. Nat. Commun. 2021, 12, 1053. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.Y.; Ho, K.C.; Chu, C.W. Highly conductive PEDOT: PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Bugárová, N.; Špitálsky, Z.; Mičušík, M.; Bodík, M.; Šiffalovič, P.; Koneracká, M.; Závišová, V.; Kubovčíková, M.; Kajanová, I.; Zaťovičová, M.; et al. A multifunctional graphene oxide platform for targeting cancer. Cancers 2019, 11, 753. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Mitropoulos, A.C.; Kyzas, G.Z. Low-cost route for top-down synthesis of over-and low-oxidized graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124928. [Google Scholar] [CrossRef]
- Gurzęda, B.; Buchwald, T.; Nocuń, M.; Bąkowicz, A.; Krawczyk, P. Graphene material preparation through thermal treatment of graphite oxide electrochemically synthesized in aqueous sulfuric acid. RSC Adv. 2017, 7, 19904–19911. [Google Scholar] [CrossRef]
- Li, K.; Han, Z.; Wang, L.; Wang, J.; Zhang, C.; Lin, J.; Luo, S.; Peng, L.; Fang, W.; Liu, Y.; et al. Wrinkling modes of graphene oxide assembled on curved surfaces. Nano Res. 2023, 16, 1801–1809. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Ravindran, A.R.; Feng, C.; Huang, S.; Wang, Y.; Zhao, Z.; Yang, J. Effects of graphene nanoplatelet size and surface area on the AC electrical conductivity and dielectric constant of epoxy nanocomposites. Polymers 2018, 10, 477. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Zhang, J. Graphene and other 2D material components dynamic characterization and nanofabrication at atomic scale. J. Nanomater. 2015, 16, 81. [Google Scholar] [CrossRef]
- Štengl, V.; Henych, J.; Bludská, J.; Ecorchard, P.; Kormunda, M. A green method of graphene preparation in an alkaline environment. Ultrason. Sonochem. 2015, 24, 65–71. [Google Scholar] [CrossRef]
- Khanra, P.; Kuila, T.; Kim, N.H.; Bae, S.H.; Yu, D.S.; Lee, J.H. Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem. Eng. J. 2012, 183, 526–533. [Google Scholar] [CrossRef]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Abdel Hamid, Z. Graphene-based nanocomposites: Synthesis, mechanical properties, and characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef]
- Priya, B.; Shandilya, P.; Raizada, P.; Thakur, P.; Singh, N.; Singh, P. Photocatalytic mineralization and degradation kinetics of ampicillin and oxytetracycline antibiotics using graphene sand composite and chitosan supported BiOCl. J. Mol. Catal. A Chem. 2016, 423, 400–413. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Dual role of glycine as a chemical functionalizer and a reducing agent in the preparation of graphene: An environmentally friendly method. J. Mater. Chem. 2012, 22, 9696–9703. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, W.; Yu, B.; Wang, Y.; Cong, C.; Yu, T. Uniform decoration of reduced graphene oxide sheets with gold nanoparticles. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Jaya, R.P. Porous concrete pavement containing nanosilica from black rice husk ash. In New Materials in Civil Engineering; Butterworth-Heinemann: Oxford, UK, 2020; pp. 493–527. [Google Scholar]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Sharma, N.; Sharma, V.; Vyas, R.; Kumari, M.; Kaushal, A.; Gupta, R.; Sharma, S.K.; Sachdev, K. A new sustainable green protocol for production of reduced graphene oxide and its gas sensing properties. J. Sci. Adv. Mater. Devices 2019, 4, 473–482. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, N.; Singh, U.; Arif, M.; Singh, A. Higher oxidation level in graphene oxide. Optik 2017, 143, 115–124. [Google Scholar] [CrossRef]
- Li, M.; Ma, Q.; Luo, A.; Hong, W. Multiple toroidal dipole symmetry-protected bound states in the continuum in all-dielectric metasurfaces. Opt. Laser Technol. 2022, 154, 108252. [Google Scholar] [CrossRef]
- Xu, B.; Zhao, X.; Li, G.; Zhang, P.; Zhao, D.; Kong, X.; Hua, R. Large spatial Goos-Hänchen shifts from quasicrystals with graphene. Results Phys. 2020, 19, 103349. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, L.; Liu, F.; Zhong, D.; Wu, M. Photonic Stopband Filters Based on Graphene-Pair Arrays. Appl. Sci. 2021, 11, 11557. [Google Scholar] [CrossRef]










| Waste Sources | Methods | Temperature | Atmosphere | Time | Graphene Derivatives | References |
|---|---|---|---|---|---|---|
| Petals of lotus and hibiscus flowers | Chemical vapor deposition (CVD) | 800–1600 °C | Air | 0.5 h | graphene | [28] |
| Newspaper | Carbonization | 450 °C | Air | 5 days | graphene | [29] |
| Chitosan | Pyrolysis, Chemical activation | 800 °C 900 °C | N2 gas | 3 h 2 h | graphene | [30] |
| Camphor leaves | Pyrolysis | 1200 °C | Nitrogen gas | 4 min | graphene | [31] |
| Wheat straw | Hydrothermal, Pyrolysis, Pyrolysis | 150 °C 800 °C 2600 °C | Air N2 gas Ar gas | 6 h 3 h | graphene | [26] |
| Oil palm leaves & Palm kernel shell | Pyrolysis | 700 °C | N2 gas | 3 h | GO | [32] |
| Oil palm fiber | CVD & Pyrolysis | 1020 °C | Ar and H2 gas | 30 min | graphene | [33] |
| Rice husks | Chemical activation | 400 °C 800 °C | Air | 2 h | graphene | [34] |
| Palm oil | Pyrolysis | 900 °C | Ar gas | 10 min | GO | [35] |
| Spruce bark | Hydrothermal Pyrolysis | 180 °C 1000 °C | Air N2 gas | 12 h 2 h | graphene | [36] |
| Mango peel | Pyrolysis | 750 °C | H2 gas Ar gas | 15 min | graphene | [37] |
| Macademia nut shell | Hydrothermal Pyrolysis | 180 °C 800 °C | Air Argon gas | 12 h 2 h | graphene | [38] |
| Soybeans | Pyrolysis | 800 °C | Nitrogen gas | 2 h | graphene | [39] |
| Empty fruit brunch | Pyrolysis Graphitization | 350 °C 900 °C | N2 gas | 2 h | graphene | [40] |
| Bengal gram bean husk | Pyrolysis | 400 °C 850 °C | Nitrogen gas | 2 h | graphene | [41] |
| Populus wood | Pyrolysis | 950 °C | Nitrogen gas | 1 h | graphene | [42] |
| Lignin biomass | Hydrothermal | 180 °C | Air | 12 h | graphene | [43] |
| Walnut shell | Pyrolysis | 700 °C | Argon gas | 4 h | graphene | [44] |
| Coconut shells, Oil palm empty fruit bunches (OPEFB), Rice husks | Carbonization | 250, 300, 350, 400, 450 °C 105 °C 250, 300, 350 °C | NaOH NaOH Air | 2 h 24 h 2.5 h | GO | [45] |
| Types of Waste | Microwave Experimental Parameters | Characterizations | Applications | References | |||
|---|---|---|---|---|---|---|---|
| Power | Time | Reagents | Frequency | ||||
| Graphite powder | 700 W | 60 s | XPS, XRD and TEM | Fuel cell catalysts | [83] | ||
| Cellulose | 950 W | 2 h | H2SO4 | - | XRD | Biobased GO Quantum dots (GOQD) | [84] |
| Sugarcane bagasse (dried) & bulk | 700 W 800 W | 2 min 10 min | H2SO4 Argon gas | 2450 MHz | FESEM, XRD, XPS and Raman spectroscopy SEM, Raman spectroscopy | Li-ion battery (LIB) | [85] [86] |
| Betalain from dragon fruit | 100 W | 10 min | - | ANOVA and BBD design matrix | Coloring food product | [87] | |
| Spent tea waste | 100-900W | 15-180 min | - | TEM, XPS and FTIR | Graphene quantum dots (GQDs) | [88] | |
| Waste palm | 700 W | 5 min | - | FESEM, XRD, XPS, TEM and Raman spectroscopy | Supercapacitor | [89] | |
| Poly (Ethylene terephthalate) | 700 W | 300 s | Iron nano-particles | 2450 MHz | XPS, Raman spectroscopy, FESEM, SEM, HRTEM and EDX | Bisphenol-A removal from contaminated water | [90] |
| Coconut shells | 800 W | 10, 20, 30, 40 min | L-ascorbic acid | 2.45 MHz | FTIR, SEM, EDAX, XRD, LCR-Meters. | Effects of microwave irradiation | [91] |
| Sorghum stalk | 700 W | 3 min | - | SEM, XRD, XPS, TEM and EDS | Supercapacitors | [92] | |
| Coconut coir and coconut shell | 12 h | - | XRD, XPS, TEM and SEM | Electrical transportation system | [93] | ||
| Bovine blood waste | 700 W | 10 min | - | XPS and Raman spectroscopy | Food industry | [94] | |
| Coconut shells | 900 W | 15 min | - | XRD and Raman spectroscopy | A hybrid gas sensor from room temperature | [95] | |
| Waste PET bottle | 600 W | 2 min | - | EDX, FTIR XRD and SEM. | Tetracyclines removal | [96] | |
| Disposable mask | - | SEM microscopy | Composite materials | [97] | |||
| Grass waste | 8 h | - | FTIR, TEM, Raman, AFM, XPS, UV-Vis and HRTEM | Nonlinear optical applications | [98] | ||
| Styrofoam waste | 1100 W | 30 min | 2.45GHz | TEM, Raman, XRD, FTIR and SAED | Nonpolar GQDs-based hydrophobic coating | [99] | |
| Bamboo waste | 2000 W | 25 min | K2CO3 | - | XRD, TEM, SEM and XPS | Biochar containing graphene (BCG) | [100] |
| Melamine sponge and arjuna bark | 700 W | 10 min | - | FTIR, XPS and TEM | Cell imaging and H2O2 sensing | [101] | |
| Toner powder waste | 350 W | 30s | - | Raman, FTIR, UV-Vis spectrometer and FETEM | Color converting film | [102] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balqis, N.; Mohamed Jan, B.; Simon Cornelis Metselaar, H.; Sidek, A.; Kenanakis, G.; Ikram, R. An Overview of Recycling Wastes into Graphene Derivatives Using Microwave Synthesis; Trends and Prospects. Materials 2023, 16, 3726. https://doi.org/10.3390/ma16103726
Balqis N, Mohamed Jan B, Simon Cornelis Metselaar H, Sidek A, Kenanakis G, Ikram R. An Overview of Recycling Wastes into Graphene Derivatives Using Microwave Synthesis; Trends and Prospects. Materials. 2023; 16(10):3726. https://doi.org/10.3390/ma16103726
Chicago/Turabian StyleBalqis, Nuralmeera, Badrul Mohamed Jan, Hendrik Simon Cornelis Metselaar, Akhmal Sidek, George Kenanakis, and Rabia Ikram. 2023. "An Overview of Recycling Wastes into Graphene Derivatives Using Microwave Synthesis; Trends and Prospects" Materials 16, no. 10: 3726. https://doi.org/10.3390/ma16103726
APA StyleBalqis, N., Mohamed Jan, B., Simon Cornelis Metselaar, H., Sidek, A., Kenanakis, G., & Ikram, R. (2023). An Overview of Recycling Wastes into Graphene Derivatives Using Microwave Synthesis; Trends and Prospects. Materials, 16(10), 3726. https://doi.org/10.3390/ma16103726