Poly(Acrylic Acid)-Sodium Alginate Superabsorbent Hydrogels Synthesized by Electron Beam Irradiation Part I: Impact of Initiator Concentration and Irradiation Dose on Structure, Network Parameters and Swelling Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
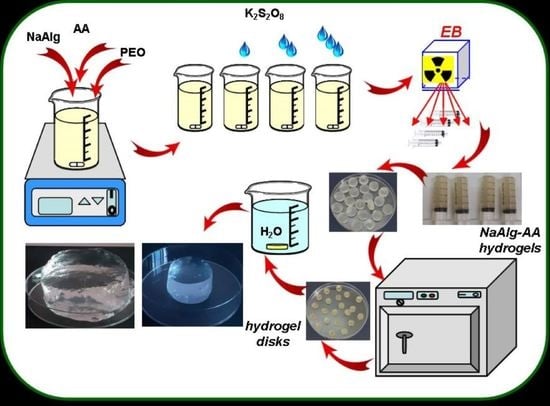
2.2. Experimental Installation and Samples Preparation
2.3. Hydrogels Characterization
2.3.1. Measurements of Gel Fraction, Swelling, and Network Parameters
2.3.2. Sol-Gel Analysis
2.3.3. Structural Investigations by Fourier-Transform Infrared Spectroscopy
2.3.4. Morphological Investigations by Scanning Electron Microscopy
3. Results and Discussion
3.1. Gel Fraction and Network Parameters
3.2. Sol-Gel Analysis Results
3.3. Structural Investigations Results (FTIR)
3.4. Morphological Investigations Results (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, Y.; Liu, X.; Li, C.; Liu, H.; Cheng, Y.; Lu, J.; Zhang, K.; Wang, H. Super-swelling lignin-based biopolymer hydrogels for soil water retention from paper industry waste. Int. J. Biol. Macromol. 2019, 135, 815–820. [Google Scholar] [CrossRef]
- González-Dugo, M.P.; Mateos, L. Spectral vegetation indices for benchmarking water productivity of irrigated cotton and sugarbeet crops. Agric. Water Manag. 2008, 95, 48–58. [Google Scholar] [CrossRef]
- Gleick, P.; Christian-Smith, J.; Cooley, H. Water-use efficiency and productivity: Rethinking the basin approach. Water Int. 2011, 36, 784–798. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop. Res. 2009, 11, 119–123. [Google Scholar] [CrossRef]
- Collins, M.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.; Culebras, M.; Teacă, C.A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D.; Wendroth, O. The Impacts of Bio-Based and Synthetic Hydrogels on Soil Hydraulic Properties: A Review. Polymers 2022, 14, 4721. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Chronopoulou, L.; Di Nitto, A.; Papi, M.; Parolini, O.; Falconi, M.; Teti, G.; Muttini, A.; Lattanzi, W.; Palmieri, V.; Ciasca, G.; et al. Biosynthesis and physico-chemical characterization of high performing peptide hydrogels@graphene oxide composites. Colloid Surf. B 2021, 207, 111989. [Google Scholar] [CrossRef]
- Amine, K.M.; Champagne, C.P.; Salmieri, S.; Britten, M.; St-Gelais, D.; Fustier, P.; Lacroix, M. Effect of palmitoylated alginate microencapsulation on viability of Bifidobacterium longum during freeze-drying. LWT Food Sci. Technol. 2014, 56, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel Application in Urban Farming: Potentials and Limitations—A Review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Faheem Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels. A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.-O.; Park, J.-S.; Kim, E.J.; Jeong, S.-I.; Lee, J.Y.; Lim, Y.-M. Preparation of Radiation Cross-Linked Poly(Acrylic Acid) Hydrogel Containing Metronidazole with Enhanced Antibacterial Activity. Int. J. Mol. Sci. 2020, 21, 187. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, N.; Jalili, L.; Anvari, F. A study on the swelling behavior of poly(acrylic acid) hydrogels obtained by electron beam crosslinking. Radiat. Phys. Chem. 2010, 79, 735–739. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulanski, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Bagher Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Mallikarjuna, B.; Madhusudana Rao, K.; Siraj, S.; Chandra Babu, A.; Chowdoji Rao, K.; Subha, M.C.S. Sodium alginate/poly (ethylene oxide) blend hydrogel membranes for controlled release of valganciclovir hydrochloride. Des. Monomers Polym. 2013, 16, 151–159. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Mehta, M.H. Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J. Control. Release 2000, 63, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Downs, E.; Robertson, N.; Riss, T.; Plunkett, M. Calcium alginate beads as a slow-release system for delivering angiogenic molecules In Vivo and In Vitro. J. Cell. Physiol. 1992, 152, 422–429. [Google Scholar] [CrossRef]
- Lin, S.Y.; Ayres, J.W. Calcium alginate beads as cone carriers of 5-aminosalysilic acid. Pharm. Res. 1992, 9, 1128–1131. [Google Scholar] [CrossRef]
- Ramesh Babu, V.; Krishna Rao, K.S.V.; Sairam, M. pH-sensitive interpenetrating network microgels of sodium alginate-acrylic acid for the controlled release of ibuprofen. J. Appl. Polym. Sci. 2006, 99, 2671–2678. [Google Scholar] [CrossRef]
- Cai, Z.; Shi, Z.; Sherman, M.; Sun, A.M. Development and of evaluation of a system of microencapsulation of primary rat hepatocytes. Hepathology 1989, 10, 855–860. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Aminabhavi, T.M. Preparation and characterization of interpenetrating network beads of poly(vinyl alcohol)-grafted-poly(acryl amide) with sodium alginate and their controlled release characteristics for cypermethrin pesticide. J. Appl. Polym. Sci. 2002, 84, 552–560. [Google Scholar] [CrossRef]
- Çaykara, T.; Demirci, S.; Eroğlu, M.S.; Güven, O. Poly(ethylene oxide) and its blends with sodium alginate. Polymer 2005, 46, 10750–10757. [Google Scholar] [CrossRef]
- Kondo, T.; Sawatari, C. Intermolecular hydrogen bonding in cellulose/poly(ethylene oxide) blends: Thermodynamic examination using 2,3-di-O- and 6-O-methylcelluloses as cellulose model compounds. Polymer 1994, 35, 4423–4428. [Google Scholar] [CrossRef]
- Erizal, E.; Wikanta, T. Synthesis of polyethylene oxide (peo)–chitosan hydrogel prepared by gamma radiation technique. Indones. J. Chem. 2011, 11, 16–20. [Google Scholar] [CrossRef]
- Sultana, S.; Islam, M.R.; Dafader, N.C.; Haque, M.E. Preparation of Carboxymethyl Cellulose/Acrylamide Copoly-Mer Hydrogel Using Gamma Radiation And Investigation of Its Swelling Behavior. J. Bangladesh Chem. Soc. 2012, 25, 132–138. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D. Swelling studies of super water retainer acrylamide/crotonic acid hydrogels crosslinked by trimethylolpropane triacrylate and 1,4-butanediol dimethacrylate. Polym. Bull. 2002, 48, 299–307. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D.; Sahiner, N.; Güven, O. Radiation induced acrylamide/citric acid hydrogelas and their swelling behaviors. J. Macromol. Sci. Part A 2001, 38, 1105–1121. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Kangwansupamonkon, W.; Chailapakul, O.; Kiatkamjornwong, S. Synthesis and swelling properties of poly[acrylamide-co-(crotonic acid)] superabsorbents. React. Funct. Polym. 2007, 67, 865–882. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Aklonis, J.J.; Salovey, R. Model filled polymers. VI. Determination of the crosslink density of polymeric beads by swelling. J. Polym. Sci. B 1991, 29, 1035–1038. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D.; Güven, O. Influence of some crosslinkers on the swelling of acrylamide-crotonic acid hydrogels. Turk. J. Chem. 1997, 21, 151–161. Available online: https://journals.tubitak.gov.tr/chem/vol21/iss3/1 (accessed on 22 May 2023).
- Thakur, A.; Wanchoo, R.K.; Singh, P. Structural Parameters and Swelling Behavior of pHSensitive Poly(acrylamide-co-acrylic acid) Hydrogels. Chem. Biochem. Eng. Q. 2011, 25, 181–194. Available online: https://hrcak.srce.hr/69850 (accessed on 22 May 2023).
- Lee, B.H.; Li, B.; Guelcher, S.A. Gel Microstructure Regulates Proliferation and Differentiation of MC3T3-E1 Cells Encapsulated in Alginate Beads. Acta Biomater. 2012, 8, 1693–1702. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Yang, M.; Wang, D.; Jing, X.; Lin, Y.; Feng, L.; Duan, X. DPD Study on the Interfacial Properties of PEO/PEO-PPO-PEO/PPO Ternary Blends: Effects of Pluronic Structure and Concentration. Polymers 2021, 13, 2866. [Google Scholar] [CrossRef]
- James, J.D.; Ludwick, J.M.; Wheeler, M.L.; Oyen, M.L. Compressive failure of hydrogel spheres. J. Mat. Res. 2020, 35, 1227–1235. [Google Scholar] [CrossRef]
- Olejniczak, J.; Rosiak, J.; Charlesby, A. Gel/Dose Curves for Polymers Undergoing Simultaneous Crosslinking and Scission. Int. J. Radiat. Appl. Instrum. C 1991, 37, 499–504. [Google Scholar] [CrossRef]
- Sedlacek, O.; Kucka, J.; Monnery, B.; Slouf, M.; Vetrik, M.; Hoogenboom, R.; Hruby, M. The effect of ionizing radiation on biocompatible polymers: From sterilization to radiolysis and hydrogel formation. Polym. Degrad. Stab. 2017, 137, 1–10. [Google Scholar] [CrossRef]
- Erizal; Sudirman; Budianto, E.; Mahendra, A.; Yudianti, R. Radiation Synthesis of Superabsorbent Poly(acrylamide-co-acrylic acid)-Sodium Alginate Hydrogels. Adv. Mat. Res. 2013, 746, 88–96. [Google Scholar] [CrossRef]
- Jing, R.; Yanqun, Z.; Jiuqiang, L.; Hongfei, H. Radiation synthesis and characteristic of IPN hydrogels composed of poly(diallyldimethylammonium chloride) and Kappa-Carrageenan. Radiat. Phys. Chem. 2001, 62, 277–281. [Google Scholar] [CrossRef]
- Demeter, M.; Calina, I.; Scarisoreanu, A.; Micutz, M. E-Beam Cross-Linking of Complex Hydrogels Formulation: The Influence of Poly(Ethylene Oxide) Concentration on the Hydrogel Properties. Gels 2022, 8, 27. [Google Scholar] [CrossRef]
- Nagasawa, N.; Mitomo, H.; Yoshii, F.; Kume, T. Radiation-induced degradation of sodium alginate. Polym. Degrad. Stab. 2000, 69, 279–285. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arab. J. Chem. 2017, 10, S431–S438. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.; Wang, A. Synthesis, characterization and swelling behaviors of sodium alginate-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2009, 75, 79–84. [Google Scholar] [CrossRef]
- Tamada, M. Radiation Processing of Polymers and Its Applications. In Radiation Applications, 1st ed.; An Advanced Course in Nuclear Engineering Book Series; Kudo, H., Ed.; Springer: Singapore, 2018; Volume 7, pp. 63–80. [Google Scholar] [CrossRef]
- Yoshi, F. Application to the Radiation Processing of Polymer. In Proceedings of the FNCA Workshop on Applications of Electron Accelerator, Takasaki, Japan, 28 January–1 February 2002; pp. 108–116. [Google Scholar]
- Jurkin, T.; Pucic, I. Poly(ethyleneoxide)irradiated in the solid state, melt and aqueous solution—A DSC and WAXD study. Radiat. Phys. Chem. 2012, 81, 1303–1308. [Google Scholar] [CrossRef]
- Mohamed, S.; Mahmoud, G.; Taleb, M.A. Synthesis and characterization of poly(acrylic acid)-g-sodium alginate hydrogel initiated by gamma irradiation for controlled release of chlortetracycline HCl. Mon. Chem. 2013, 144, 129–137. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Ighigeanu, D. New Type of Sodium Alginate-g-acrylamide Polyelectrolyte Obtained by Electron Beam Irradiation: Characterization and Study of Flocculation Efficacy and Heavy Metal Removal Capacity. Polymers 2019, 11, 234. [Google Scholar] [CrossRef] [Green Version]
- Bardajee, G.; Hooshyar, Z.; Zehtabi, F.; Pourjavadi, A. A superabsorbent hydrogel network based on poly ((2-dimethylaminoethyl) methacrylate) and sodium alginate obtained by c-radiation: Synthesis and characterization. Iran. Polym. J. 2012, 21, 829–836. [Google Scholar] [CrossRef]
- Chang, K.A.; Chew, L.Y.; Law, K.P.; Ng, J.F.; Wong, C.S.; Wong, C.L.; Hussein, S. Effect of gamma irradiation on the physicochemical properties of sodium alginate solution and internally crosslinked film made thereof. Radiat. Phys. Chem. 2022, 193, 09963. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Ghasemzadeh, H.; Hosseinzadeh, H. Preparation and swelling behaviour of a novel anti-salt superabsorbent hydrogel based on kappa-carrageenan and sodium alginate grafted with polyacrylamide. e-Polymers 2004, 4, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y. Relationship between Water Absorbency and Reaction Conditions in Aqueous Solution Polymerization of Polyacrylate Superabsorbents. J. Appl. Polym. Sci. 2000, 75, 808–814. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, M.; Shen, Y. Enhanced swelling ratio and water retention capacity for novel superabsorbent hydrogel. Colloid Surf. A 2019, 583, 123972. [Google Scholar] [CrossRef]
- Manaila, E.; Demeter, M.; Calina, I.C.; Craciun, G. NaAlg-g-AA Hydrogels: Candidates in Sustainable Agriculture Applications. Gels 2023, 9, 316. [Google Scholar] [CrossRef]
- Kadłubowski, S.; Henke, A.; Ulański, P.; Rosiak, J.M. Hydrogels of Polyvinylpyrrolidone (PVP) and Poly(Acrylic Acid) (PAA) Synthesized by Radiation-Induced Crosslinking of Homopolymers. Radiat. Phys. Chem. 2010, 79, 261–266. [Google Scholar] [CrossRef]
- Mozalewska, W.; Czechowska-Biskup, R.; Olejnik, A.K.; Wach, R.A.; Ulański, P.; Rosiak, J.M. Chitosan-Containing Hydrogel Wound Dressings Prepared by Radiation Technique. Radiat. Phys. Chem. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Ulański, P.; Bothe, E.; Hildenbrand, K.; Von Sonntag, C.; Rosiak, J.M. The Influence of Repulsive Electrostatic Forces on the Lifetimes of Poly(Acrylic Acid) Radicals in Aqueous Solution. Nukleonika 1997, 42, 425–436. [Google Scholar]
- Makuuchi, K.; Cheng, S. Radiation Processing of Aqueous Polymer Systems. In Radiation Processing of Polymer Materials and Its Industrial Applications; Wiley: Hoboken, NJ, USA, 2011; pp. 276–299. ISBN 9781118162798. [Google Scholar]
- Sadeghi, M.; Godarzi, A.; Khani, F.; Mirdarikvande, S.; Sadeghi, H.; Shasavari, H. Synthesis of a Novel Biopolymer-Based Alginate Superabsorbent Hydrogel. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 169–174. [Google Scholar]
- Pucic, I.; Jurkin, T. FTIR Assessment of Poly(Ethylene Oxide) Irradiated in Solid State, Melt and Aqeuous Solution. Radiat. Phys. Chem. 2012, 81, 1426–1429. [Google Scholar] [CrossRef]
- Yi, X.; Xu, Z.; Liu, Y.; Guo, X.; Minrui, O.; Xu, X. Highly Efficient Removal of Uranium(VI) from Wastewater by Polyacrylic Acid Hydrogels. RSC Adv. 2017, 7, 6278–6287. [Google Scholar] [CrossRef] [Green Version]
- Coletta, C.; Cui, Z.; Dazzi, A.; Guigner, J.M.; Néron, S.; Marignier, J.L.; Remita, S. A Pulsed Electron Beam Synthesis of PEDOT Conducting Polymers by Using Sulfate Radicals as Oxidizing Species. Radiat. Phys. Chem. 2016, 126, 21–31. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An Interpenetrating Alginate/Gelatin Network for Three-Dimensional (3D) Cell Cultures and Organ Bioprinting. Molecules 2020, 25, 756. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zhang, X.; Deng, X.; Hao, L.; Wang, W. Modification of Alginate Hydrogel Films for Delivering Hydrophobic Kaempferol. J. Nanomater. 2019, 2019, 9170732. [Google Scholar] [CrossRef]












| Irradiation Dose (kGy) | PP, K2S2O8, Concentration (%) | |||
|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | |
| Mc (kg/mol) | ||||
| 5 | 719.0 ± 52.7 | 825.0 ± 42.8 | 545.8 ± 35.7 | 348.1 ± 21.7 |
| 10 | 375.6 ± 30.5 | 294.6 ± 31.6 | 273.0 ± 18.9 | 296.0 ± 27.0 |
| 15 | 160.8 ± 14.9 | 109.2 ± 7.9 | 137.9 ± 12.9 | 147.2 ± 11.9 |
| 20 | 31.9 ± 3.0 | 48.2 ± 5.9 | 45.7 ± 4.0 | 54.2 ± 4.6 |
| υ2,s × 103 (cm3) | ||||
| 5 | 2.1 ± 0.09 | 1.9 ± 0.06 | 2.4 ± 0.09 | 3.0 ± 0.11 |
| 10 | 3.3 ± 0.16 | 3.4 ± 0.22 | 3.5 ± 0.14 | 3.5 ± 0.19 |
| 15 | 5.2 ± 0.28 | 6.5 ± 0.28 | 5.6 ± 0.31 | 5.4 ± 0.26 |
| 20 | 13.4 ± 0.73 | 10.5 ± 0.76 | 10.8 ± 0.56 | 9.8 ± 0.49 |
| P (%) | ||||
| 5 | 99.7 ± 0.01 | 99.8 ± 0.01 | 99.7 ± 0.01 | 99.7 ± 0.01 |
| 10 | 99.5 ± 0.02 | 99.6 ± 0.03 | 99.6 ± 0.02 | 99.5 ± 0.03 |
| 15 | 99.2 ± 0.04 | 99.2 ± 0.03 | 99.3 ± 0.04 | 99.3 ± 0.03 |
| 20 | 98.3 ± 0.09 | 98.6 ± 0.10 | 98.6 ± 0.07 | 98.8 ± 0.06 |
| Irradiation Dose (kGy) | PP (K2S2O8) Concentration (%) | |||
|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | |
| 5 | 34,423 ± 1509 | 42,954 ± 1333 | 32,854 ± 1285 | 29,130 ± 1088 |
| 10 | 20,469 ± 994 | 23,197 ± 1490 | 24,338 ± 1004 | 21,331 ± 1163 |
| 15 | 13,189 ± 729 | 13,105 ± 712 | 13,960 ± 779 | 14,879 ± 717 |
| 20 | 5922 ± 330 | 7206 ± 528 | 7360 ± 387 | 8040 ± 408 |
| K2S2O8 | p0/q0 | Dg (kGy) | Dv (kGy) | R2 |
|---|---|---|---|---|
| 0% | 0.20 | 1.20 | −0.52 | 0.92 |
| 0.1% | 0.27 | 0.97 | −0.51 | 0.99 |
| 0.2% | 0.34 | 2.96 | −2.90 | - |
| 0.3% | 0.32 | 0.59 | 0 | - |
| K2S2O8 (%) | GX, GS × 10−9 mol/J | GS:GX (20 kGy) | |||
|---|---|---|---|---|---|
| 5 kGy | 10 kGy | 15 kGy | 20 kGy | ||
| 0 | 2.04 1/0.83 2 | 1.80 1/0.73 2 | 2.85 1/1.16 2 | 12.60 1/5.15 2 | 0.40 |
| 0.1 | 1.95 1/1.08 2 | 3.17 1/1.75 2 | 5.23 1/2.89 2 | 8.01 1/4.42 2 | 0.55 |
| 0.2 | 2.92 1/2.01 2 | 3.14 1/2.16 2 | 3.89 1/2.68 2 | 8.94 1/6.16 2 | 0.68 |
| 0.3 | 5.06 1/3.29 2 | 2.60 1/1.69 2 | 3.75 1/2.44 2 | 7.47 1/4.56 2 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craciun, G.; Calina, I.C.; Demeter, M.; Scarisoreanu, A.; Dumitru, M.; Manaila, E. Poly(Acrylic Acid)-Sodium Alginate Superabsorbent Hydrogels Synthesized by Electron Beam Irradiation Part I: Impact of Initiator Concentration and Irradiation Dose on Structure, Network Parameters and Swelling Properties. Materials 2023, 16, 4552. https://doi.org/10.3390/ma16134552
Craciun G, Calina IC, Demeter M, Scarisoreanu A, Dumitru M, Manaila E. Poly(Acrylic Acid)-Sodium Alginate Superabsorbent Hydrogels Synthesized by Electron Beam Irradiation Part I: Impact of Initiator Concentration and Irradiation Dose on Structure, Network Parameters and Swelling Properties. Materials. 2023; 16(13):4552. https://doi.org/10.3390/ma16134552
Chicago/Turabian StyleCraciun, Gabriela, Ion Cosmin Calina, Maria Demeter, Anca Scarisoreanu, Marius Dumitru, and Elena Manaila. 2023. "Poly(Acrylic Acid)-Sodium Alginate Superabsorbent Hydrogels Synthesized by Electron Beam Irradiation Part I: Impact of Initiator Concentration and Irradiation Dose on Structure, Network Parameters and Swelling Properties" Materials 16, no. 13: 4552. https://doi.org/10.3390/ma16134552
APA StyleCraciun, G., Calina, I. C., Demeter, M., Scarisoreanu, A., Dumitru, M., & Manaila, E. (2023). Poly(Acrylic Acid)-Sodium Alginate Superabsorbent Hydrogels Synthesized by Electron Beam Irradiation Part I: Impact of Initiator Concentration and Irradiation Dose on Structure, Network Parameters and Swelling Properties. Materials, 16(13), 4552. https://doi.org/10.3390/ma16134552