Eco-Friendly Polysaccharide-Based Synthesis of Nanostructured MgO: Application in the Removal of Cu2+ in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
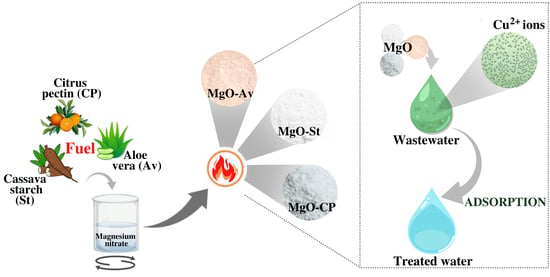
2.1. Preparation of Magnesium Oxide
2.2. Synthesis 1—Cassava Starch (MgO-St)
2.3. Synthesis 2—Citric Pectin (MgO-CP)
2.4. Synthesis 3—Aloe vera (MgO-Av)
2.5. Adsorption Tests
2.6. Characterization Techniques
3. Results
3.1. Characterization of Synthesized MgO Particles
3.1.1. X-ray Diffractometry (XRD)
3.1.2. Morphological Analysis (SEM)
3.1.3. Textural Properties (BET and BJH Isotherms)
3.1.4. Zeta Potential (ζ) vs. pH
4. Copper Ion Removal by MgO as Adsorbent
4.1. Adsorption Isotherms
4.2. Adsorption Mechanisms
4.2.1. Energy Dispersive Spectroscopy (EDS)
4.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adriano, D.C. Trace Elements in the Terrestrial Environment; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 3540961585. [Google Scholar]
- Abraham, M.R.; Susan, T.B. Water Contamination with Heavy Metals and Trace Elements from Kilembe Copper Mine and Tailing Sites in Western Uganda; Implications for Domestic Water Quality. Chemosphere 2017, 169, 281–287. [Google Scholar] [CrossRef]
- Dias, C.A.; Da Costa, A.S.V.; Guedes, G.R.; Umbelino, G.J.d.M.; de Sousa, L.G.; Alves, J.H.; Silva, T.G.M. Impactos Do Rompimento Da Barragem de Mariana Na Qualidade Da Água Do Rio Doce/Samarco’sdam Failure and Its Consequences for the Quality of the Doce River Water. Rev. Espinhaço 2018, 7, 21–35. [Google Scholar]
- Gabriel, F.Â.; Hauser-Davis, R.A.; Soares, L.; Mazzuco, A.C.A.; Rocha, R.C.C.; Pierre, T.D.S.; Saggioro, E.; Correia, F.V.; Ferreira, T.O.; Bernardino, A.F. Contamination and Oxidative Stress Biomarkers in Estuarine Fish Following a Mine Tailing Disaster. PeerJ 2020, 8, e10266. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of Using Green Adsorbent of Heavy Metal Removal from Aqueous Solutions: Adsorption Kinetics, Isotherm, Thermodynamic, Mechanism and Economic Analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Bhagat, B.; Jadeja, V.; Sharma, P.; Bandyopadhyay, R.; Mukherjee, K. Glycine Mediated Colorimetric Method along with Image Analyses Algorithms for Detection of Cu (II) in Water. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 286, 116027. [Google Scholar] [CrossRef]
- Dermentzis, K.; Christoforidis, A.; Valsamidou, E. Removal of Nickel, Copper, Zinc and Chromium from Synthetic and Industrial Wastewater by Electrocoagulation. Int. J. Environ. Sci. 2011, 1, 697–710. [Google Scholar]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of Iron, Aluminium, Manganese and Copper from Leach Solutions of Lithium-Ion Battery Waste Using Ion Exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Duarte-Nass, C.; Rebolledo, K.; Valenzuela, T.; Kopp, M.; Jeison, D.; Rivas, M.; Azócar, L.; Torres-Aravena, Á.; Ciudad, G. Application of Microbe-Induced Carbonate Precipitation for Copper Removal from Copper-Enriched Waters: Challenges to Future Industrial Application. J. Environ. Manag. 2020, 256, 109938. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, T.; Qiu, Z.; Liu, H.; Xia, Y.; Wang, Z.; Zou, R.; Liu, C. Enhanced Lead and Copper Removal in Wastewater by Adsorption onto Magnesium Oxide Homogeneously Embedded Hierarchical Porous Biochar. Bioresour. Technol. 2022, 365, 128146. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yin, H.; Zhu, M.; Yu, X.; Shao, P.; Dang, Z. MgO-Loaded Nitrogen and Phosphorus Self-Doped Biochar: High-Efficient Adsorption of Aquatic Cu2+, Cd2+, and Pb2+ and Its Remediation Efficiency on Heavy Metal Contaminated Soil. Chemosphere 2022, 294, 133733. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Jobara, A.; Bekouche, H.; Abd Allateef, M.; Ben Aissa, M.A.; Modwi, A. Impact of Cu Ions Removal onto MgO Nanostructures: Adsorption Capacity and Mechanism. J. Mater. Sci. Mater. Electron. 2022, 33, 12500–12512. [Google Scholar] [CrossRef]
- Dhal, J.P.; Sethi, M.; Mishra, B.G.; Hota, G. MgO Nanomaterials with Different Morphologies and Their Sorption Capacity for Removal of Toxic Dyes. Mater. Lett. 2015, 141, 267–271. [Google Scholar] [CrossRef]
- Ge, L.; Wang, W.; Peng, Z.; Tan, F.; Wang, X.; Chen, J.; Qiao, X. Facile Fabrication of Fe@MgO Magnetic Nanocomposites for Efficient Removal of Heavy Metal Ion and Dye from Water. Powder Technol. 2018, 326, 393–401. [Google Scholar] [CrossRef]
- Lv, G.; Zhu, C.; Zhang, H.; Su, Y.; Qian, P. Mechanism of CO2 Adsorption on Point-Defective MgO Surfaces: First-Principles Study. Appl. Surf. Sci. 2022, 604, 154647. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Y.; Wen, X.; Cheng, S.; Li, J.; Lin, Q. Key Roles of the Crystal Structures of MgO-Biochar Nanocomposites for Enhancing Phosphate Adsorption. Sci. Total Environ. 2021, 766, 142618. [Google Scholar] [CrossRef] [PubMed]
- Bassioni, G.; Farid, R.; Mohamed, M.; Hammouda, R.M.; Kühn, F.E. Effect of Different Parameters on Caustic Magnesia Hydration and Magnesium Hydroxide Rheology: A Review. Mater. Adv. 2021, 2, 6519–6531. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Fabrication of MgO Nanostructures and Its Efficient Photocatalytic, Antibacterial and Anticancer Performance. J. Photochem. Photobiol. B Biol. 2019, 190, 8–20. [Google Scholar] [CrossRef]
- Primo, J.d.O.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide Nanoparticles by Ecofriendly Routes: Adsorbent for Copper Removal From Wastewater. Front. Chem. 2020, 8, 571790. [Google Scholar] [CrossRef]
- Mastuli, M.S.; Kamarulzaman, N.; Nawawi, M.A.; Mahat, A.M.; Rusdi, R.; Kamarudin, N. Growth Mechanisms of MgO Nanocrystals via a Sol-Gel Synthesis Using Different Complexing Agents. Nanoscale Res. Lett. 2014, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Lei, R.; Zhang, T.C.; Du, D.; Zhan, W. Insight into the Role and Mechanism of Polysaccharide in Polymorphous Magnesium Oxide Nanoparticle Synthesis for Arsenate Removal. Chemosphere 2022, 296, 133878. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Araujo, E.M.; Melo, J.J.A. Biopolímeros, Polímeros Biodegradáveis e Polímeros Verdes, Revista Eletrônica de Materiais e Processos. Rev. Eletrôn. Mater. Process. 2011, 6, 127–139. [Google Scholar]
- Dalpasquale, M.; Mariani, F.Q.; Müller, M.; Anaissi, F.J. Citrus Pectin as a Template for Synthesis of Colorful Aluminates. Dyes Pigments 2016, 125, 124–131. [Google Scholar] [CrossRef]
- Choi, S.; Chung, M.H. A Review on the Relationship between Aloe Vera Components and Their Biologic Effects. Semin. Integr. Med. 2003, 1, 53–62. [Google Scholar] [CrossRef]
- Primo, J.d.O.; Borth, K.W.; Peron, D.C.; Teixeira, V.d.C.; Galante, D.; Bittencourt, C.; Anaissi, F.J. Synthesis of Green Cool Pigments (CoxZn1-XO) for Application in NIR Radiation Reflectance. J. Alloys Compd. 2019, 780, 17–24. [Google Scholar] [CrossRef]
- O’Brien, R.W.; Hunter, R.J. The Electrophoretic Mobility of Large Colloidal Particles. Can. J. Chem. 1981, 59, 1878–1887. [Google Scholar] [CrossRef]
- Moulavi, M.H.; Kale, B.B.; Bankar, D.; Amalnerkar, D.P.; Vinu, A.; Kanade, K.G. Green Synthetic Methodology: An Evaluative Study for Impact of Surface Basicity of MnO2 Doped MgO Nanocomposites in Wittig Reaction. J. Solid State Chem. 2019, 269, 167–174. [Google Scholar] [CrossRef]
- Prado, D.C.; Fernández, I.; Rodríguez-Páez, J.E. MgO Nanostructures: Synthesis, Characterization and Tentative Mechanisms of Nanoparticles Formation. Nano-Struct. Nano-Objects 2020, 23, 100482. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Chen, R.; Yang, C.; Xiang, L.; Yi, M. A Vacuum Calcination Route to High-Surface-Area MgO Nanoplates for Superior Arsenate Adsorption and Catalytic Properties. Vacuum 2018, 158, 231–235. [Google Scholar] [CrossRef]
- Ananda, A.; Ramakrishnappa, T.; Archana, S.; Yadav, L.S.R.; Shilpa, B.M.; Nagaraju, G.; Jayanna, B.K. Materials Today: Proceedings Green Synthesis of MgO Nanoparticles Using Phyllanthus Emblica for Evans Blue Degradation and Antibacterial Activity. Mater. Today Proc. 2022, 49, 801–810. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Isabel Alvarez Acevedo, N.; Cristina Guimarães Rocha, M.; Carlos Bertolino Professor, L. Determinação Da Área Superficial Específica e Da Porosidade de Duas Amostras de Argilas Provenientes Da Bacia de Taubaté- São Paulo/Determination of Specific Surface Area and Porosity of Two Clays Originated from Taubaté- Basin—São Paulo. Braz. Appl. Sci. Rev. 2021, 5, 39–57. [Google Scholar] [CrossRef]
- Phillips, V.A.; Kolbe, J.L.; Opperhauser, H. Effect of PH on the Growth of Mg(OH)2 Crystals in an Aqueous Environment at 60 °C. J. Cryst. Growth 1977, 41, 228–234. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Villalba, J.C.; Santos, M.J.; Melquiades, F.L.; Anaissi, F.J. Smectitic Clays Enriched with Ferric Ions for the Rapid Removal of Anionic Dyes in Aqueous Media. Clay Miner. 2020, 55, 12–23. [Google Scholar] [CrossRef]
- Seif, S.; Marofi, S.; Mahdavi, S. Removal of Cr3+ Ion from Aqueous Solutions Using MgO and Montmorillonite Nanoparticles. Environ. Earth Sci. 2019, 78, 377. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S. Adsorption Study of Copper (II) by Chemically Modified Orange Peel. J. Hazard. Mater. 2009, 164, 1286–1292. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.S. The Hydrolysis of Cations. John Wiley & Sons, New York, London, Sydney, Toronto 1976. 489 Seiten, Preis: £ 18.60. In Berichte der Bunsengesellschaft für Physikalische Chemie; John Wiley & Sons: Hoboken, NJ, USA, 1977; Volume 81, pp. 245–246. [Google Scholar] [CrossRef]
- Yan, H.; Bai, J.; Chen, X.; Wang, J.; Zhang, H.; Liu, Q.; Zhang, M.; Liu, L. High U(vi) Adsorption Capacity by Mesoporous Mg(OH)2 Deriving from MgO Hydrolysis. RSC Adv. 2013, 3, 23278–23289. [Google Scholar] [CrossRef]
- Xu, C.; Shi, S.; Wang, X.; Zhou, H.; Wang, L.; Zhu, L.; Zhang, G.; Xu, D. Electrospun SiO2-MgO Hybrid Fibers for Heavy Metal Removal: Characterization and Adsorption Study of Pb(II) and Cu(II). J. Hazard. Mater. 2019, 381, 120974. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, Isotherms and Thermodynamic Modeling of Liquid Phase Adsorption of Rhodamine B Dye onto Raphia Hookerie Fruit Epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yang, Z.H.; Yan, L.Ü.J.; Alhassan, S.I.; Gang, H.Y.; Wang, T.; Wang, H.Y. Preparation of 2D Carbon Ribbon/Al2O3 and Nitrogen-Doped Carbon Ribbon/Al2O3 by Using MOFs as Precursors for Removing High-Fluoride Water. Trans. Nonferrous Met. Soc. China 2021, 31, 2174–2188. [Google Scholar] [CrossRef]
- Said, K.A.M.; Ismail, N.Z.; Jama’in, R.L.; Alipah, N.A.M.; Sutan, N.M.; Gadung, G.G.; Baini, R.; Zauzi, N.S.A. Application of Freundlich and Temkin Isotherm to Study the Removal of Pb(II) via Adsorption on Activated Carbon Equipped Polysulfone Membrane. Int. J. Eng. Technol. 2018, 7, 91–93. [Google Scholar] [CrossRef]
- Moscofian, A.S.O.; Airoldi, C. Synthesized Layered Inorganic-Organic Magnesium Organosilicate Containing a Disulfide Moiety as a Promising Sorbent for Cations Removal. J. Hazard. Mater. 2008, 160, 63–69. [Google Scholar] [CrossRef]
- Ciesielczyk, F.; Bartczak, P.; Klapiszewski, Ł.; Jesionowski, T. Treatment of Model and Galvanic Waste Solutions of Copper(II) Ions Using a Lignin/Inorganic Oxide Hybrid as an Effective Sorbent. J. Hazard. Mater. 2017, 328, 150–159. [Google Scholar] [CrossRef]
- Cao, C.Y.; Qu, J.; Wei, F.; Liu, H.; Song, W.G. Superb Adsorption Capacity and Mechanism of Flowerlike Magnesium Oxide Nanostructures for Lead and Cadmium Ions. ACS Appl. Mater. Interfaces 2012, 4, 4283–4287. [Google Scholar] [CrossRef]
- Li, N.; Dang, H.; Chang, Z.; Zhao, X.; Zhang, M.; Li, W.; Zhou, H.; Sun, C. Synthesis of Uniformly Distributed Magnesium Oxide Micro-/Nanostructured Materials with Deep Eutectic Solvent for Dye Adsorption. J. Alloys Compd. 2019, 808, 151571. [Google Scholar] [CrossRef]
- Wang, X.; Cai, W.; Lin, Y.; Wang, G.; Liang, C. Mass production of micro/nanostructured porous ZnO plates and their strong structurally enhanced and selective adsorption performance for environmental remediation. J. Mater. Chem. 2010, 20, 8582–8590. [Google Scholar] [CrossRef]
- Wu, P.Y.; Jiang, Y.P.; Zhang, Q.Y.; Jia, Y.; Peng, D.Y.; Xu, W. Comparative Study on Arsenate Removal Mechanism of MgO and MgO/TiO2 Composites: FTIR and XPS Analysis. New J. Chem. 2016, 40, 2878–2885. [Google Scholar] [CrossRef]
- Siddqui, N.; Sarkar, B.; Pendem, C.; Khatun, R.; Sivakumar Konthala, L.N.; Sasaki, T.; Bordoloi, A.; Bal, R. Highly Selective Transfer Hydrogenation of α,β-Unsaturated Carbonyl Compounds Using Cu-Based Nanocatalysts. Catal. Sci. Technol. 2017, 7, 2828–2837. [Google Scholar] [CrossRef]
- Shakir, I.; Almutairi, Z.; Shar, S.S.; Nafady, A. Fabrication of a Flower-like Cu(OH)2 Nanoarchitecture and Its Composite with CNTs for Use as a Supercapacitor Electrode. Ceram. Int. 2022, 48, 11278–11285. [Google Scholar] [CrossRef]
- Rhatigan, S.; Nolan, M. Impact of Surface Hydroxylation in MgO-/SnO-Nanocluster Modified TiO2 Anatase (101) Composites on Visible Light Absorption, Charge Separation and Reducibility. Chin. Chem. Lett. 2018, 29, 757–764. [Google Scholar] [CrossRef]







| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (Å) | Volume (cc/g) |
|---|---|---|---|---|
| MgO-St | 43.93 | 0.130 | 20.06 | 89.76 |
| MgO-CP | 7.01 | 0.015 | 16.29 | 11.01 |
| MgO-Av | 6.63 | 0.009 | 16.29 | 6.44 |
| Adsorption Solutions (g·L−1) | pH |
|---|---|
| 0.95 | 3.77 |
| 1.6 | 3.54 |
| 3.0 | 3.27 |
| 5.4 | 3.11 |
| 6.4 | 3.07 |
| 11 | 2.88 |
| Samples | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL (L·mg−1) | qmax (mg·g−1) | RL2 | KF (L·g−1) | 1/n | RF2 | KT (L·g−1) | b (J·mol−1) | RT2 | |
| MgO-St | 1.779 | 6331.117 | 0.589 | 3974.443 | 1.103 | 0.999 | 76.831 | 2.777 | 0.725 |
| MgO-Av | 2.386 | 5831.244 | 0.817 | 4125.036 | 1.683 | 0.989 | 40.953 | 1.840 | 0.987 |
| MgO-CP | 1.356 | 6726.623 | 0.547 | 3895.705 | 1.500 | 0.991 | 44.904 | 2.546 | 0.733 |
| Adsorbent | Adsorption Capacities qmax (mg·g−1) | References |
|---|---|---|
| Magnesium phyllosilicate modified with 2-aminophenyldisufide | 208.44 | (Moscofian and Airoldi, 2008) [43] |
| Lignin/MgO-SiO2 | 83.98 | (Ciesielczyk et al., 2017b) [44] |
| Electrospun SiO2-MgO hybrid fibers | 493.00 | (Xu et al., 2019) [39] |
| MgO nanostructures | 546.45 | (Ismail et al., 2022) [12] |
| Samples | Mg% | O% | C% | Cu% | S% |
|---|---|---|---|---|---|
| MgO-St | 37.01 | 51.00 | 11.99 | - | - |
| Mg-St1 | 11.66 | 47.97 | 19.75 | 18.24 | 2.38 |
| Mg-St2 | 0.44 | 41.66 | 8.34 | 42.91 | 6.66 |
| MgO-Av | 50.06 | 44.86 | 5.08 | - | - |
| Mg-Av1 | 16.43 | 44.78 | 11.66 | 23.99 | 3.14 |
| Mg-Av2 | 1.04 | 41.11 | 11.14 | 39.62 | 7.09 |
| MgO-CP | 41.55 | 48.41 | 10.04 | - | - |
| Mg-CP1 | 12.75 | 48.50 | 19.11 | 17.60 | 2.04 |
| Mg-Cp2 | 0.20 | 40.65 | 13.47 | 39.44 | 6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaba, N.; Horsth, D.F.L.; Correa, J.d.S.; Primo, J.d.O.; Jaerger, S.; Alves, H.J.; Bittencourt, C.; Anaissi, F.J. Eco-Friendly Polysaccharide-Based Synthesis of Nanostructured MgO: Application in the Removal of Cu2+ in Wastewater. Materials 2023, 16, 693. https://doi.org/10.3390/ma16020693
Balaba N, Horsth DFL, Correa JdS, Primo JdO, Jaerger S, Alves HJ, Bittencourt C, Anaissi FJ. Eco-Friendly Polysaccharide-Based Synthesis of Nanostructured MgO: Application in the Removal of Cu2+ in Wastewater. Materials. 2023; 16(2):693. https://doi.org/10.3390/ma16020693
Chicago/Turabian StyleBalaba, Nayara, Dienifer F. L. Horsth, Jamille de S. Correa, Julia de O. Primo, Silvia Jaerger, Helton J. Alves, Carla Bittencourt, and Fauze J. Anaissi. 2023. "Eco-Friendly Polysaccharide-Based Synthesis of Nanostructured MgO: Application in the Removal of Cu2+ in Wastewater" Materials 16, no. 2: 693. https://doi.org/10.3390/ma16020693
APA StyleBalaba, N., Horsth, D. F. L., Correa, J. d. S., Primo, J. d. O., Jaerger, S., Alves, H. J., Bittencourt, C., & Anaissi, F. J. (2023). Eco-Friendly Polysaccharide-Based Synthesis of Nanostructured MgO: Application in the Removal of Cu2+ in Wastewater. Materials, 16(2), 693. https://doi.org/10.3390/ma16020693