Recovery of Chlorosilane Residual Liquid to Prepare Nano-Silica via the Reverse Micro-Emulsion Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
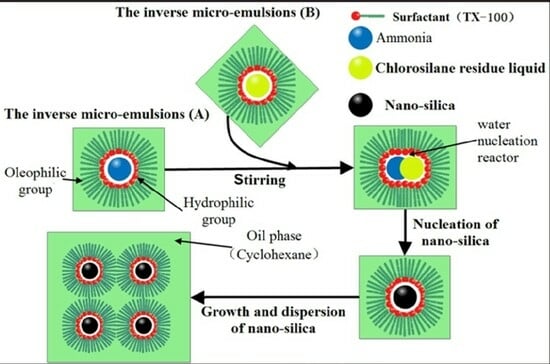
2.2. Preparation of Silica
2.2.1. Preparation Method of Silica
2.2.2. Effect of Reaction Conditions on Silica
2.2.3. Particle Characterization
3. Results and Discussion
3.1. Effect of Different Reaction Components on Silica
3.1.1. Effect of Chlorosilane Residue Liquid Concentration on the Particle Size and Morphology of Nano-Silica
3.1.2. Influence of Different HLB Values on the Particle Size and Morphology of Nano-Silica
3.1.3. Effect of Different Molar Ratios of Water and Surfactant (ω) on the Particle Size and Morphology of Nano-Silica
3.1.4. Influence of Ammonia Concentration on the Particle Size and Morphology of Nano-Silica
3.2. Characterization of Nano-Silica Performance
3.2.1. Phase Analysis of Nano-Silica
3.2.2. FT-IR Analysis of Nano-Silica
3.2.3. Pore Structure Analysis of Nano-Silica
3.2.4. Thermal Stability Analysis of Nano-Silica
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, L.; Noor, T.; Iqbal, N. A comprehensive and critical review of the recent progress in electrocatalysts for the ethanol oxidation reaction. RSC Adv. 2021, 11, 16768–16804. [Google Scholar] [CrossRef] [PubMed]
- Marupi, M.; Batool, M.; Alizadeh, M.; Zanib, N. Transient Stability Improvement of Large-Scale Photovoltaic Grid Using a Flywheel as a Synchronous Machine. Energies 2023, 16, 689. [Google Scholar] [CrossRef]
- Che Mat, A.N.; Jefrey Basirun, W.; Asrina Sairi, N.; Shahid Mehmood, M. Photoelectrochemical reduction of CO2 over Ru/Mn/Co trimetallic catalysts supported anatase TiO2 under visible light irradiation. J. Sol-Gel Sci. Technol. 2020, 94, 279–287. [Google Scholar] [CrossRef]
- Pi, S.; Li, H. Vertical cooperation and coopetition of incumbents under the new energy substitute: Evidence from Chinese automobile industry. J. Clean. Prod. 2022, 359, 131964. [Google Scholar] [CrossRef]
- Liu, Y.; Han, L.; Xu, Y. The impact of geopolitical uncertainty on energy volatility. Int. Rev. Financ. Anal. 2021, 75, 101743. [Google Scholar] [CrossRef]
- Raduwan, N.F.; Shaari, N.; Kamarudin, S.K.; Masdar, M.S.; Yunus, R.M. An overview of nanomaterials in fuel cells: Synthesis method and application. Int. J. Hydrogen Energy 2022, 47, 18468–18495. [Google Scholar] [CrossRef]
- Kafle, B.; Mack, S.; Temann, C.; Bashardoust, S.; Clochard, L.; Duffy, E.; Rentsch, J. Atmospheric pressure dry etching of polysilicon layers for highly reverse bias-stable topcon solar cells. Sol. RRL 2022, 6, 2100481. [Google Scholar] [CrossRef]
- Potapov, V.; Fediuk, R.; Gorev, D. Obtaining sols, gels and mesoporous nanopowders of hydrothermal nanosilica. J. Sol-Gel Sci. Technol. 2020, 94, 681–694. [Google Scholar] [CrossRef]
- Xu, B.; Su, Y.; Chen, L.; Cai, J.; Huang, B. Preparation of mesoporous silica nanoparticles with controlled pore size, particle diameter, morphology, and structure by two-step process of chlorosilane residue. Ceram. Int. 2018, 44, 22241–22248. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Niu, L.; Zou, X.; Zhang, C.; Ruan, X. A field experiment on stabilization of Cd in contaminated soils by surface-modified nano-silica (SMNS) and its phyto-availability to corn and wheat. J. Soils Sediments 2020, 20, 91–98. [Google Scholar] [CrossRef]
- Singh, J.; Boddula, R.; Digambar Jirimali, H. Utilization of secondary agricultural products for the preparation of value added silica materials and their important applications: A review. J. Sol-Gel Sci. Technol. 2020, 96, 15–33. [Google Scholar] [CrossRef]
- Mohammadikish, M.; Masteri-Farahani, M.; Mahdian, T. Optical properties of copper tungstate nanoparticles prepared by microemulsion method. Inorg. Nano-Met. Chem. 2019, 49, 63–68. [Google Scholar] [CrossRef]
- Mahtabani, A.; La Zara, D.; Anyszka, R.; He, X.; Paajanen, M.; Van Ommen, J.R.; Blume, A. Gas phase modification of silica nanoparticles in a fluidized bed: Tailored deposition of aminopropylsiloxane. Langmuir 2021, 37, 4481–4492. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, J.; Yan, F.; Tian, S.; Li, K. A novel low temperature vapor phase hydrolysis method for the production of nano-structured silica materials using silicon tetrachloride. RSC Adv. 2014, 4, 8703–8710. [Google Scholar] [CrossRef]
- Farhan, R.Z.; Ebrahim, S.E. Preparing nanosilica particles from rice husk using precipitation method. Baghdad Sci. J. 2021, 18, 494–500. [Google Scholar] [CrossRef]
- Daud, F.; Azmy, N.; Mahmud, M.S.; Sariffudin, N.; Zaki, H. Preparation of Nanosilica Powder Using Rice Husk via Precipitation Method. Mater. Sci. Forum 2020, 1010, 501–507. [Google Scholar] [CrossRef]
- Nguyen, X.H.; Tran, N.A.; Nguyen, T.T.H.; Dao, T.T.N. Nanosilica synthesis and application for lead treatment in water. J. Vietnam. Environ. 2018, 9, 255–263. [Google Scholar] [CrossRef]
- Stopic, S.; Wenz, F.; Husovic, T.V.; Friedrich, B. Synthesis of silica particles using ultrasonic spray pyrolysis method. Metals 2021, 11, 463. [Google Scholar] [CrossRef]
- Gebretatios, A.G.; Pillantakath, A.R.K.K.; Witoon, T.; Lim, J.W.; Banat, F.; Cheng, C.K. Rice husk waste into various template-engineered mesoporous silica materials for different applications: A comprehensive review on recent developments. Chemosphere 2022, 310, 136843–136867. [Google Scholar] [CrossRef] [PubMed]
- Sk, A.; Sk, N.M.; Konda, R.K.; Naik, V. Micro Emulsions: An Overview and Pharmaceutical Applications. World J. Curr. Med. Pharm. Res. 2020, 2, 201–205. [Google Scholar]
- Sun, B.; Chai, J.; Chai, Z.; Zhang, X.; Cui, X.; Lu, J. A surfactant-free microemulsion consisting of water, ethanol, and dichloromethane and its template effect for silica synthesis. J. Colloid Interface Sci. 2018, 526, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Kang, W.; Yang, H.; Li, Z.; Zhou, B.; He, Y.; Wang, J.; Aidarova, S.; Sarsenbekuly, B. Advances of microemulsion and its applications for improved oil recovery. Adv. Colloid Interface Sci. 2021, 299, 102527. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Huang, B.; Zhang, J.; Hu, X.; Li, Y.; Xue, J. Resource utilization of chlorosilane residual liquid to prepare nano-silica in reverse microemulsion system. J. Mater. Sci. Mater. Electron. 2020, 31, 11317–11324. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, Y. Preparation of spherical silica particles in reverse micro emulsions using silicon tetrachloride as precursor. J. Non-Cryst. Solids 2012, 358, 337–341. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Luo, G.; Bai, S. Using hydrolysis of silicon tetrachloride to prepare highly dispersed precipitated nanosilica. Chem. Eng. J. 2016, 283, 1–8. [Google Scholar] [CrossRef]
- Pinto, P.R.; Mendes, L.C.; Dias, M.L.; Azuma, C. Synthesis of acrylic-modified sol-gel silica. Colloid Polym. Sci. 2006, 284, 529–535. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ciriminna, R.; Ilharco, L.M.; Pagliaro, M. Role of the alkyl-alkoxide precursor on the structure and catalytic properties of hybrid sol-gel catalysts. Chem. Mater. 2005, 17, 6686–6694. [Google Scholar] [CrossRef]
- Pijarn, N.; Jaroenworaluck, A.; Sunsaneeyametha, W.; Stevens, R. Synthesis and characterization of nanosized-silica gels formed under controlled conditions. Powder Technol. 2010, 203, 462–468. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Tian, S.; Liu, Z.; Shi, J.; Li, K.; Chen, X.; Xu, Y. A green and facile synthesis of ordered mesoporous nanosilica using coal fly ash. ACS Sustain. Chem. Eng. 2016, 4, 654–4661. [Google Scholar] [CrossRef]
- Cerveny, S.; Schwartz, G.A.; Otegui, J.; Colmenero, J.; Loichen, J.; Westermann, S. Dielectric study of hydration water in silica nanoparticles. J. Phys. Chem. C 2012, 116, 4340–24349. [Google Scholar] [CrossRef]












| No. | TX-100/n-Hexanol (Mass Ratio) | Ammonia/Cyclohexane (Mass Ratio) | Concentration of Chlorosilane Residue Liquid (mol/L) |
|---|---|---|---|
| a | 3:2 | 1:10 | 0.04 |
| b | 3:2 | 1:10 | 0.08 |
| c | 3:2 | 1:10 | 0.12 |
| d | 3:2 | 1:10 | 0.16 |
| e | 3:2 | 1:10 | 0.20 |
| No. | TX-100/n-Hexanol (Mass Ratio) | Ammonia/Cyclohexane (Mass Ratio) | HLB Value |
|---|---|---|---|
| a | 9:0 | 1:10 | 13.50 |
| b | 3:1 | 1:10 | 11.63 |
| c | 3:2 | 1:10 | 10.50 |
| d | 1:1 | 1:10 | 9.75 |
| e | 3:4 | 1:10 | 9.21 |
| No. | TX-100/n-Hexanol (Mass Ratio) | Ammonia/Cyclohexane (Mass Ratio) | Mass of Distilled Water (g) | Molar Ratio of Water and Surfactant (ω) |
|---|---|---|---|---|
| a | 3:2 | 1:15 | 0 | 9 |
| b | 3:2 | 1:15 | 0.75 | 12 |
| c | 3:2 | 1:15 | 1.75 | 16 |
| d | 3:2 | 1:15 | 2.75 | 20 |
| e | 3:2 | 1:15 | 5.75 | 32 |
| No. | TX-100/n-Hexanol (Mass Ratio) | Ammonia/Cyclohexane (Mass Ratio) | Mass of Distilled Water (g) | Concentration of Ammonium Hydroxide (mol/L) | Molar Ratio of Water and Surfactant (ω) |
|---|---|---|---|---|---|
| a | 3:2 | 1:45 | 4.25 | 0.15 | 20 |
| b | 3:2 | 1:22.5 | 3.5 | 0.3 | 20 |
| c | 3:2 | 1:15 | 2.75 | 0.44 | 20 |
| d | 3:2 | 1:11.25 | 2 | 0.58 | 20 |
| e | 3:2 | 1:10 | 1.625 | 0.65 | 20 |
| Silicon Source | Method | Particle Size | The Specific Surface Area | References |
|---|---|---|---|---|
| Chlorosilane residual liquid | Inverse micro-emulsions | 33.4 nm | 850.5 m2/g | This study |
| Silicon tetrachloride | Low temperature vapor phase hydrolysis | 141.7 nm | 418 m2/g | [14] |
| Rice husk | Precipitation method | 52.83 nm | 618 m2/g | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Li, Y.; Wei, L.; Xue, J.; Lin, N.; Zha, X.; Fang, G. Recovery of Chlorosilane Residual Liquid to Prepare Nano-Silica via the Reverse Micro-Emulsion Process. Materials 2023, 16, 6912. https://doi.org/10.3390/ma16216912
Cai J, Li Y, Wei L, Xue J, Lin N, Zha X, Fang G. Recovery of Chlorosilane Residual Liquid to Prepare Nano-Silica via the Reverse Micro-Emulsion Process. Materials. 2023; 16(21):6912. https://doi.org/10.3390/ma16216912
Chicago/Turabian StyleCai, Jixiang, Youwen Li, Lianghuan Wei, Jiangpeng Xue, Ning Lin, Xianghao Zha, and Guodong Fang. 2023. "Recovery of Chlorosilane Residual Liquid to Prepare Nano-Silica via the Reverse Micro-Emulsion Process" Materials 16, no. 21: 6912. https://doi.org/10.3390/ma16216912
APA StyleCai, J., Li, Y., Wei, L., Xue, J., Lin, N., Zha, X., & Fang, G. (2023). Recovery of Chlorosilane Residual Liquid to Prepare Nano-Silica via the Reverse Micro-Emulsion Process. Materials, 16(21), 6912. https://doi.org/10.3390/ma16216912