Solid State Processing of BCZT Piezoceramics Using Ultra Low Synthesis and Sintering Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
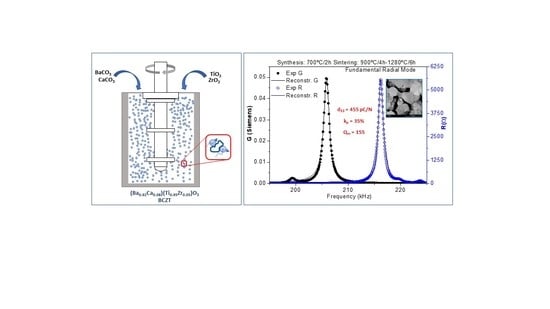
2.2. Powder and Ceramics Processing
2.3. Structural and Microstructural Characterization
2.4. Dielectric and Electromechanical Characterization
 ), accounts for the validity of the material model for the resonance mode. The closer is the model to the experimental curves; the closer is
), accounts for the validity of the material model for the resonance mode. The closer is the model to the experimental curves; the closer is  to 1. For the planar mode, the complex material coefficients (P* = P′ − iP′′) directly determined in this analysis are the piezoelectric charge coefficient, d31, the dielectric permittivity, εT33, and the elastic compliances, sE11 and sE12. Additionally, a few other material coefficients are determined by the software from those, using well known relationships. These allow us to analyze the performances of the ceramic as generator (piezoelectric voltage coefficient g31) and as energy transducer (electromechanical coupling factors (Kp,K31)and frequency number (Np = fs(kHz). D(mm), where D is the diameter of the disk)). Losses can be expressed for each material complex coefficient as loss tangent factor (tanδ = P′′/P′), commonly used for the dielectric coefficients, or as a quality factor (Q = P′/P′′), commonly used for the elastic coefficients.
to 1. For the planar mode, the complex material coefficients (P* = P′ − iP′′) directly determined in this analysis are the piezoelectric charge coefficient, d31, the dielectric permittivity, εT33, and the elastic compliances, sE11 and sE12. Additionally, a few other material coefficients are determined by the software from those, using well known relationships. These allow us to analyze the performances of the ceramic as generator (piezoelectric voltage coefficient g31) and as energy transducer (electromechanical coupling factors (Kp,K31)and frequency number (Np = fs(kHz). D(mm), where D is the diameter of the disk)). Losses can be expressed for each material complex coefficient as loss tangent factor (tanδ = P′′/P′), commonly used for the dielectric coefficients, or as a quality factor (Q = P′/P′′), commonly used for the elastic coefficients.3. Results and Discussion
3.1. Processing and Characterization of the Powders
3.1.1. Thermal Analysis of the Powders
3.1.2. X-ray Diffraction of the Powders
3.2. Processing and Characterization of the Sintered Disks
3.2.1. X-ray Diffraction of the Sintered Disks
3.2.2. SEM Analysis of the Sintered Ceramics
3.3. Electrical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCabe, E.E.; Bousquet, E.; Stockdale, C.P.J.; Deacon, C.A.; Tran, T.T.; Halasyamani, P.S.; Stennett, M.C.; Hyatt, N.C. Proper ferroelectricity in the Dion–Jacobson material CsBi2Ti2NbO10: Experiment and Theory. Chem. Mat. 2015, 27, 8298–8309. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, M.; Jiménez, F.J.; Pardo, L.; Ochoa, P.; González, A.M.; de Frutos, J. A New Prospect in Road Traffic Energy Harvesting Using Lead-Free Piezoceramics. Materials 2019, 12, 3725. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gou, G.; Li, J. Ruddlesden–Popper perovskite sulfides A3B2S7: A new family of ferroelectric photovoltaic materials for the visible spectrum. Nano Energy 2016, 22, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Wendari, T.P.; Arief, S.; Mufti, N.; Blake, G.R.; Baas, J.; Suendo, V.; Prasetyo, A.; Insani, A.; Zulhadjri, Z. Lead-Free Aurivillius Phase Bi2LaNb1.5Mn0.5O9: Structure, Ferroelectric, Magnetic, and Magnetodielectric Effects. Inorg. Chem. 2022, 61, 8644–8652. [Google Scholar] [CrossRef]
- Garroni, S.; Senes, N.; Iacomini, A.; Enzo, S.; Mulas, G.; Pardo, L.; Cuesta-Lopez, S. Advanced Synthesis on Lead-Free KxNa(1−x)NbO3 Piezoceramics for Medical Imaging Applications. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rödel, J.; Webber, K.G.; Dittmer, R.; Jo, W.; Kimura, M.; Damjanovic, D. Transferring Lead-Free Piezoelectric Ceramics into Application. J. Eur. Ceram. Soc. 2015, 35, 1659–1681. [Google Scholar] [CrossRef]
- McQuarrie, M.; Behnke, F.W. Structural and Dielectric Studies in the System (Ba, Ca) (Ti, Zr)O3. J. Am. Ceram. Soc. 1954, 37, 539–543. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X. Large Piezoelectric Effect in Pb-Free Ceramics. Phys. Rev. Lett. 2009, 103, 257602. [Google Scholar] [CrossRef] [Green Version]
- Villafuerte-Castrejón, M.E.; Morán, E.; Reyes-Montero, A.; Vivar-Ocampo, R.; Peña-Jiménez, J.A.; Rea-López, S.O.; Pardo, L. Towards Lead-Free Piezoceramics: Facing a Synthesis Challenge. Materials 2016, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Hayati, R.; Fayazi, M.; Diargar, H.; Kaveh, M.; Tayebi, L. Electrical and Mechanical Properties of BZT−XBCT Lead-Free Piezoceramics. Int. J. Appl. Ceram. Technol. 2020, 17, 1891–1898. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Zang, G. High Piezoelectric d33 Coefficient of Lead-Free (Ba0.93 Ca0.07)(Ti0.95 Zr0.05)O3 Ceramics Sintered at Optimal Temperature. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 65–67. [Google Scholar] [CrossRef]
- Xiao, A.; Xie, X.; He, L.; Yang, Y.; Ji, Y. Enhanced Piezoelectric Properties in a Single-Phase Region of Sm-Modified Lead-Free (Ba,Ca)(Zr,Ti)O3 Ceramics. Materials 2022, 15, 7839. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Wu, W.; Chen, Q.; Zhu, J.; Yang, Z.; Wang, J. Composition and Poling Condition-Induced Electrical Behavior of (Ba0.85 Ca0.15)(Ti1-X Zrx)O3 Lead-Free Piezoelectric Ceramics. J. Eur. Ceram. Soc. 2012, 32, 891–898. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Chen, W. A Brief Review of Ba(Ti0.8Zr0.2)O3-(Ba0.7Ca0.3)TiO3 Based Lead-Free Piezoelectric Ceramics: Past, Present and Future Perspectives. J. Phys. Chem. Solids 2018, 114, 207–219. [Google Scholar] [CrossRef]
- Bai, Y.; Matousek, A.; Tofel, P.; Bijalwan, V.; Nan, B.; Hughes, H.; Button, T.W. (Ba,Ca)(Zr,Ti)O3 Lead-Free Piezoelectric Ceramics-The Critical Role of Processing on Properties. J. Eur. Ceram. Soc. 2015, 35, 3445–3456. [Google Scholar] [CrossRef]
- Reyes-Montero, A.; Pardo, L.; López-Juárez, R.; González, A.M.; Cruz, M.P.; Villafuerte-Castrejón, M.E. Lead-Free Ba0.9Ca0.1Ti0.9Zr 0.1O3 Piezoelectric Ceramics Processed below 1300 °C. J. Alloys Compd. 2014, 584, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, J.; Chao, X.; Wei, L.; Yang, B.; Wang, D.; Yang, Z. Synthesis, Structure, Dielectric, Piezoelectric, and Energy Storage Performance of (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3 Ceramics Prepared by Different Methods. J. Mater. Sci. Mater. Electron. 2016, 27, 5047–5058. [Google Scholar] [CrossRef]
- Hunpratub, S.; Maensiri, S.; Chindaprasirt, P. Synthesis and Characterization of Ba0.85Ca0.15Ti 0.9Zr0.1O3 Ceramics by Hydrothermal Method. Ceram. Int. 2014, 40, 13025–13031. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Tang, X.G.; Ju, S.G.; Liu, Q.X.; Zhang, T.F.; Xiong, H.F. Influence of Sintering Temperature on the Ferroelectric and Piezoelectric Properties of (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 Ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 3048–3052. [Google Scholar] [CrossRef]
- Keeble, D.S.; Benabdallah, F.; Thomas, P.A.; Maglione, M.; Kreisel, J. Revised Structural Phase Diagram of (Ba0.7Ca 0.3TiO3)-(BaZr0.2Ti0.8O3). Appl. Phys. Lett. 2013, 102, 092903. [Google Scholar] [CrossRef]
- Reyes-Montero, A.; Pardo, L.; López-Juárez, R.; González, A.M.; Rea-López, S.O.; Cruz, M.P.; Villafuerte-Castrejón, M.E. Sub-10 µm Grain Size, Ba1-XCaxTi0.9Zr0.1O3 (x = 0.10 and x = 0.15) Piezoceramics Processed Using a Reduced Thermal Treatment. Smart Mater. Struct. 2015, 24, 065033. [Google Scholar] [CrossRef]
- Amorín, H.; Venet, M.; Chinarro, E.; Ramos, P.; Algueró, M.; Castro, A. Lead-Free Ba0.85Ca0.15Zr0.1Ti0.9O3 Ferroelectric Ceramics with Refined Microstructure and High Strain under Electric Field by Mechanosynthesis. J. Eur. Ceram. Soc. 2022, 42, 4907–4916. [Google Scholar] [CrossRef]
- Damjanovic, D.; Biancoli, A.; Batooli, L.; Vahabzadeh, A.; Trodahl, J. Elastic, dielectric, and piezoelectric anomalies and Raman spectroscopy of 0.5Ba(Ti0.8 Zr0. 2)O3-0.5(Ba0. 7 Ca0. 3) TiO3. Appl. Phys. Lett. 2012, 100, 192907. [Google Scholar] [CrossRef] [Green Version]
- Pisitpipathsin, N.; Kantha, P. Ferroelectric and Piezoelectric Properties of Ba0.85Ca0.15Zr0.1Ti0.9O3 Ceramic with Various Sintering Times. Integr. Ferroelectr. 2018, 187, 138–146. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Zhou, C.; Xu, R.; Zhang, S.; Li, Z.; Gao, R.; Fu, C. Electric Fatigue of BCZT Ceramics Sintered in Different Atmospheres. Appl. Phys. A Mater. Sci. Process. 2019, 125, 759. [Google Scholar] [CrossRef]
- Thanaboonsombut, A.; Vaneesorn, N. Effect of Attrition Milling on the Piezoelectric Properties of Bi 0.5Na0.5TiO3-Based Ceramics. J. Electroceramics 2008, 21, 414–417. [Google Scholar] [CrossRef]
- Mittal, S.; Pahi, S.; Singh, K.C. Size Effect of Nano-Scale Powders on the Microstructure and Electrical Properties of Ba0.85Ca0.15Zr0.1Ti0.9O3 Ceramics. J. Mater. Sci. Mater. Electron. 2019, 30, 15493–15503. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Martín-Gonzalez, M.S.; Fernández, J.F. Effect of Stoichiometry and Milling Processes in the Synthesis and the Piezoelectric Properties of Modified KNN Nanoparticles by Solid-state Reaction. J. Eur. Ceram. Soc. 2010, 30, 2763–2771. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Marcos, F.; Ochoa, P.; Fernandez, J.F. Sintering and Properties of Lead-Free (K,Na,Li)(Nb,Ta,Sb)O3 Ceramics. J. Eur. Ceram. Soc. 2007, 27, 4125–4129. [Google Scholar] [CrossRef]
- Zuow, R.; Rödel, J.; Chen, R.; Li, L. Sintering and Electrical Properties of Lead-Free Na0.5K 0.5NbO3 Piezoelectric Ceramics. J. Am. Ceram. Soc. 2006, 89, 2010–2015. [Google Scholar] [CrossRef]
- Juhyun, Y.; Min, S.; Hong, J.; Suh, S.; Ur, S. Microstructural and Piezoelectric Characteristics of PSN-PMN-PZT Ceramics Produced by Attriction Milling. Trans. Electr. Electron. Mater. 2001, 2, 18–23. [Google Scholar]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Zang, G. Piezoelectric and Dielectric Properties of (Ba1-XCa x)(Ti0.95Zr0.05)O3 Lead-Free Ceramics. J. Am. Ceram. Soc. 2010, 93, 2942–2944. [Google Scholar] [CrossRef]
- Thakur, O.P.; Feteira, A.; Kundys, B.; Sinclair, D.C. Influence of Attrition Milling on the Electrical Properties of Undoped-BaTiO3. J. Eur. Ceram. Soc. 2007, 27, 2577–2589. [Google Scholar] [CrossRef]
- Alemany, C.; González, A.M.; Pardo, L.; Jiménez, B.; Carmona, F.; Mendiofa, J. Automatic Determination of Complex Constants of Piezoelectric Lossy Materials in the Radial Mode. J. Phys. D. Appl. Phys. 1995, 28, 945–956. [Google Scholar] [CrossRef]
- Yang, R.B.; Fu, W.G.; Deng, X.Y.; Tan, Z.W.; Zhang, Y.J.; Han, L.R.; Lu, C.; Guan, X.F. Preparation and characterization of (Ba0.88Ca0.12) (Zr0.12Ti0.88) O3 powders and ceramics produced by sol-gel process. Adv Mat Res. 2010, 148–149, 1062–1066. [Google Scholar] [CrossRef]
- Ji, X.; Wang, C.; Li, S.; Zhang, S.; Tu, R.; Shen, Q.; Shi, J.; Zhang, L. Structural and electrical properties of BCZT ceramics synthesized by sol–gel process. J. Mater. Sci. Mater. Electron. 2018, 29, 7592–7599. [Google Scholar] [CrossRef]
- Ciomaga, C.E.; Curecheriu, L.P.; Lukacs, V.A.; Horchidan, N.; Doroftei, F.; Valois, R.; Mitoseriu, L. Optimization of Processing Steps for Superior Functional Properties of (Ba, Ca)(Zr, Ti) O3. Ceramics Mater. 2022, 15, 8809. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovicová, M.; Baláž, M.; Billik, P.; Zara, C.Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of Mechanochemistry: From Nanoparticles to Technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [Green Version]
- Nanni, P.; Leoni, M.; Buscaglia, V.; Aliprandi, G. Low-temperature aqueous preparation of barium metatitanate powders. J.Eur.Soc. 1994, 14, 85–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.-J.; Chen, W. Li-modified Ba0. 99Ca0. 01Zr0. 02Ti0. 98O3 lead-free ceramics with highly improved piezoelectricity. J. Alloy. Compd. 2017, 694, 745–751. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Zang, G. Polymorphic phase transition and piezoelectric properties of (Ba1’xCax)(Ti0.9Zr0.1)O3 lead-free ceramics. Phys. B 2010, 405, 4513–4516. [Google Scholar] [CrossRef]








| Sintering Conditions | Cell Parameters (Å) c | Cell Parameters (Å) a | Tetragonal Distortion c/a | Main Perovskite Phase G.P. |
|---|---|---|---|---|
| 900 °C/ 1 h + 1280 °C/2 h | 4.0237 | 4.0030 | 1.005 | P4mm BCZT |
| 900 °C/ 1 h + 1280 °C/4 h | 4.0306 | 4.0090 | 1.005 | P4mm BCZT |
| 900 °C/2 h + 1280 °C/4 h | 4.0216 | 4.0011 | 1.005 | P4mm BCZT |
| 900 °C/2 h + 1280 °C/6 h | 4.0143 | 3.9978 | 1.004 | P4mm BCZT |
| 900 °C/3 h + 1280 °C/6 h | 4.0235 | 4.0099 | 1.003 | Single Phase P4mm BCZT |
| 900 °C/4 h + 1280 °C/6 h | 4.0205 | 4.0030 | 1.004 | Single Phase P4mm BCZT |
| Properties\ Sintering Conditions | 1150 °C 1 h (1 BM) | 1260 °C 30 min (2 BM) | 1280 °C 2 h (2 BM) | 1280 °C 4 h (2 BM) | 1300 °C 4 h (2 BM) |
|---|---|---|---|---|---|
| Density (g/cc) | 4.34 | 4.60 | 5.02 | 4.78 | 4.41 |
| Resistance (MΩ) | 0.6 | 0.4 | 2 | 30 | 15 |
| /tanδ (at 1kHz) [1] | 1671 0.264 | 3396 0.543 | 2306 0.286 | 1903 0.159 | 2463 0.175 |
| d33 (pC/N)[2] | 38 | 52 | 185 | 189 | 125 |
| Properties/ Sintering | 900 °C/1 h 1280 °C/2 h | 900 °C/1 h 1280 °C/4 h | 900 °C/2 h 1280 °C/6 h | 900 °C/3 h 1280 °C/6 h | 900 °C/4 h 1280 °C/6 h |
|---|---|---|---|---|---|
| Density (g/cc) | 4.30 | 4.45 | 4.47 | 4.32 | 4.28 |
| Resistance (MΩ) | 5 | 8 | 9 | 1 | 2 |
| /tanδ (at 1 kHz) [1] | 2100 0.326 | 2014 0.282 | 2158 0.262 | 3052 0.511 | 2833 0.251 |
| d33 (pC/N) [2] | 145 | 200 | 140 | 405 | 455 |
| Properties\ Sintering Conditions | 1150 °C 1 h (1 BM) | 1280 °C 2 h | 1280 °C 4 h | 900 °C 1 h 1280 °C 4 h | 900 °C 3 h 1280 °C 6 h | 900 °C 4 h 1280 °C 6 h | 1450 °C 3 h [2] |
|---|---|---|---|---|---|---|---|
 | 0.9996 | 0.9997 | 0.9995 | 0.9998 | 0.9999 | 0.9975 | 0.9964 |
| kp (%) | 4.65 | 15.36 | 19.02 | 23.23 | 29.31 | 35.12 | 27.82 |
| Np(kHz.mm) | 2652 | 2740 | 2874 | 2897 | 2339 | 2559 | 2742 |
| d′31 (pC/N) | −9.89 | −36.3 | −45.0 | −55.7 | −99.6 | −108.8 | −68.17 |
| Qp(d31) | 79 | 38 | 49 | 46 | 49 | 21 | 130 |
| / tanδ | 947 0.026 | 1458 0.029 | 1542 0.022 | 1498 0.021 | 1897 0.020 | 1797 0.078 | 1540 0.013 |
| g′31 (pC/N) | −1.18 | −2.81 | −3.30 | −4.20 | −5.93 | −6.81 | −5.0 |
| [1] (1010N m−2) | 7.36 | 8.71 | 8.93 | 8.41 | 5.40 | 6.57 | 8.40 |
| Qm | 208 | 162 | 197 | 188 | 120 | 155 | 157 |
| Synthesis Method | Synthesis T (°C) | Sintering T (°C) | d33 (pC/N) | Composition | Reference |
|---|---|---|---|---|---|
| SSR | 1200 | 1450 | 365 | BCZT0805 | [32] |
| SSR | 1100 | 1250 | 340 | Li-modified BCZT0102 | [40] |
| SSR | 1350 | 1450 1500 | 620 | BCZT1510 | [8] |
| SSR | 1250 | 1420 | 406 | BCZT1510 | [17] |
| SSR | 1250 | 1400 | 410 | BCZT1510 | [21] |
| SSR | 1250 | 1400 | 300 | BCZT1010 | [21] |
| SSR | 1300 | 1500 | 330 | BCZT1510 | [10] |
| SSR | 1200 | 1450 | 328 | BCZT1610 | [41] |
| Mechano-activation | 900 | 1450 | 270 | BCZT1510 | [22] |
| Sol-gel | 1000 | 1420 | 540 | BCZT1510 | [17] |
| Pechini | 700 | 1275 | 390 | BCZT1010 | [16] |
| Hydrothermal | 240 | 1300 | 164 | BCZT1510 | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureddu, M.; Bartolomé, J.F.; Lopez-Esteban, S.; Dore, M.; Enzo, S.; García, Á.; Garroni, S.; Pardo, L. Solid State Processing of BCZT Piezoceramics Using Ultra Low Synthesis and Sintering Temperatures. Materials 2023, 16, 945. https://doi.org/10.3390/ma16030945
Mureddu M, Bartolomé JF, Lopez-Esteban S, Dore M, Enzo S, García Á, Garroni S, Pardo L. Solid State Processing of BCZT Piezoceramics Using Ultra Low Synthesis and Sintering Temperatures. Materials. 2023; 16(3):945. https://doi.org/10.3390/ma16030945
Chicago/Turabian StyleMureddu, Marzia, José F. Bartolomé, Sonia Lopez-Esteban, Maria Dore, Stefano Enzo, Álvaro García, Sebastiano Garroni, and Lorena Pardo. 2023. "Solid State Processing of BCZT Piezoceramics Using Ultra Low Synthesis and Sintering Temperatures" Materials 16, no. 3: 945. https://doi.org/10.3390/ma16030945
APA StyleMureddu, M., Bartolomé, J. F., Lopez-Esteban, S., Dore, M., Enzo, S., García, Á., Garroni, S., & Pardo, L. (2023). Solid State Processing of BCZT Piezoceramics Using Ultra Low Synthesis and Sintering Temperatures. Materials, 16(3), 945. https://doi.org/10.3390/ma16030945