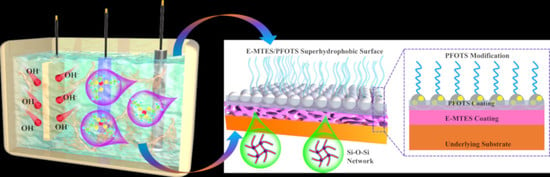
Investigation of Electrochemical Assisted Deposition of Sol-Gel Silica Films for Long-Lasting Superhydrophobicity
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Electrodeposition of E-MTES Films
2.3. Preparation of Superhydrophobic Composite Coating
2.4. Characterization
3. Results and Discussion
3.1. Process Control of the EAD of E-MTES Coatings
3.2. Surface Microstructure and Coating Thickness by EAD
3.3. Chemical Analyses of the Coated Surfaces
3.4. Surface 3D Profile and Roughness
3.5. Water Contact Angle (WCA) Measurements and Stability Tests
3.6. Self-Cleaning Test and Durability Evaluation
3.7. Mechanisms of Chemical Bonding and Durability of Superhydrophobicity
3.8. Comparison with Other Research Work in Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of electroplating industry wastewater: A review on the various techniques. Environ. Sci. Pollut. Res. 2022, 29, 72196–72246. [Google Scholar] [CrossRef]
- Yu, X.; Hou, Y.; Ren, X.; Sun, C.; Wang, M. Research progress on the removal, recovery and direct high-value materialization of valuable metal elements in electroplating/electroless plating waste solution. J. Water Process Eng. 2022, 46, 102577. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Rodak, D.E.; Wong, C.A.; Hayden, C.A. Effects of micro- and nano-structures on the self-cleaning behaviour of lotus leaves. Nanotechnology 2006, 17, 1359–1362. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Aishwarya, S.; Shanthi, J. Spin-coated polymer composite hydrophobic surfaces with self-cleaning performance. Mater. Rese. Express 2019, 6, 076412. [Google Scholar] [CrossRef]
- Raimondo, M.; Veronesi, F.; Boveri, G.; Guarini, G.; Motta, A.; Zanoni, R. Superhydrophobic properties induced by sol-gel routes on copper surfaces. Appl. Surf. Sci. 2017, 422, 1022–1029. [Google Scholar] [CrossRef]
- Varshney, P.; Mohapatra, S.S. Durable and regenerable superhydrophobic coatings for brass surfaces with excellent self-cleaning and anti-fogging properties prepared by immersion technique. Tribol. Int. 2018, 123, 17–25. [Google Scholar] [CrossRef]
- Tuo, Y.; Zhang, H.; Rong, W.; Jiang, S.; Chen, W.; Liu, X. Drag Reduction of Anisotropic Superhydrophobic Surfaces Prepared by Laser Etching. Langmuir 2019, 35, 11016–11022. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.T.; Xu, S.; Yang, H.; Sun, H.B. Bioinspired Superhydrophobic Surfaces via Laser-Structuring. Front. Chem. 2020, 8, 835. [Google Scholar] [CrossRef]
- Hasan, J.; Xu, Y.; Yarlagadda, T.; Schuetz, M.; Spann, K.; Yarlagadda, P.K. Antiviral and Antibacterial Nanostructured Surfaces with Excellent Mechanical Properties for Hospital Applications. ACS Biomater. Sci. Eng. 2020, 6, 3608–3618. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Das, R.; Hamid, S.B.A.; Hashim, N.A.; AlOmar, M.K.; Alayan, H.M.; Novikov, M.; Alsalhy, Q.F.; Hashim, M.A. Optimization of the Synthesis of Superhydrophobic Carbon Nanomaterials by Chemical Vapor Deposition. Sci. Rep. 2018, 8, 2778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhou, H.; Wan, S.; Cai, G.-Y.; Dong, Z.-H. Fabrication of superhydrophobic coating on magnesium alloy with improved corrosion resistance by combining micro-arc oxidation and cyclic assembly. Surf. Coat. Technol. 2018, 339, 155–166. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Yi, L.; Yan, X.; Li, J. Fabricating superhydrophobic and oleophobic surface with silica nanoparticles modified by silanes and environment-friendly fluorinated chemicals. Appl. Surf. Sci. 2018, 450, 102–111. [Google Scholar] [CrossRef]
- Sebastian, D.; Yao, C.-W.; Lian, I. Abrasion Resistance of Superhydrophobic Coatings on Aluminum Using PDMS/SiO2. Coatings 2018, 8, 414. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, Q.; Chen, Q.; Yin, H.; Wang, S.; Hu, C. Preparation of TiO2 Superhydrophobic Composite Coating and Studies on Corrosion Resistance. Front. Chem. 2022, 10, 943055. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Li, S.; Liu, J.; Han, Z.; Ren, L. Biomimetic superhydrophobic surface of high adhesion fabricated with micronano binary structure on aluminum alloy. ACS Appl. Mater. Interfaces 2013, 5, 8907–8914. [Google Scholar] [CrossRef]
- Hashjin, R.R.; Ranjbar, Z.; Yari, H.; Momen, G. Tuning up sol-gel process to achieve highly durable superhydrophobic coating. Surf. Interfaces 2022, 33, 102282. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Shi, J.; Shen, B.; Huang, J.; Hu, J.; Chen, Z.; Lai, Y. A superhydrophobic TPU/CNTs@SiO2 coating with excellent mechanical durability and chemical stability for sustainable anti-fouling and anti-corrosion. Chem. Eng. J. 2022, 434, 134605. [Google Scholar] [CrossRef]
- Lv, Z.; Yu, S.; Song, K.; Zhou, X.; Yin, X. A two-step method fabricating a hierarchical leaf-like superamphiphobic PTFE/CuO coating on 6061Al. Prog. Org. Coat. 2020, 147, 105723. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Mahadik, S.S. Surface morphological and topographical analysis of multifunctional superhydrophobic sol-gel coatings. Ceram. Int. 2021, 47, 29475–29482. [Google Scholar] [CrossRef]
- Vidal, K.; Gómez, E.; Goitandia, A.M.; Angulo-Ibáñez, A.; Aranzabe, E. The Synthesis of a Superhydrophobic and Thermal Stable Silica Coating via Sol-Gel Process. Coatings 2019, 9, 627. [Google Scholar] [CrossRef]
- Balaji, J.; Raja, P.B.; Sethuraman, M.G.; Oh, T.H. Recent studies on sol–gel based corrosion protection of Cu—A review. J. Sol-Gel Sci. Technol. 2022, 103, 12–38. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Pedraza, F.; Mahadik, S.S. Comparative studies on water repellent coatings prepared by spin coating and spray coating methods. Prog. Org. Coat. 2017, 104, 217–222. [Google Scholar] [CrossRef]
- Du, T.; Li, H.; Sant, G.; Bauchy, M. New insights into the sol-gel condensation of silica by reactive molecular dynamics simulations. J. Chem. Phys. 2018, 148, 234504. [Google Scholar] [CrossRef]
- Pan, G.; Schaefer, D.W. Morphology and water-barrier properties of silane films on aluminum and silicon. Thin Solid Films 2006, 503, 259–267. [Google Scholar] [CrossRef]
- Giordano, G.; Durante, C.; Gennaro, A.; Guglielmi, M. Multilayer Deposition of Silica Sol–Gel Films by Electrochemical Assisted Techniques. J. Phys. Chem. C 2016, 120, 28820–28824. [Google Scholar] [CrossRef]
- Shacham, R.; Avnir, D.; Mandler, D. Electrodeposition of Methylated Sol-Gel Films on Conducting Surfaces. Adv. Mater. 1999, 11, 384–388. [Google Scholar] [CrossRef]
- Collinson, M.M.; Higgins, D.A.; Kommidi, R.; Campbell-Rance, D. Electrodeposited Silicate Films: Importance of Supporting Electrolyte. Anal. Chem. 2008, 80, 651–656. [Google Scholar] [CrossRef]
- Liu, L.; Walcarius, A. Kinetics of the electrochemically-assisted deposition of sol-gel films. Phys. Chem. Chem. Phys. 2017, 19, 14972–14983. [Google Scholar] [CrossRef]
- Fang, L.; He, Q.-Q.; Zhou, M.-J.; Zhao, J.-P.; Hu, J.-M. Electrochemically assisted deposition of sol–gel films on graphene nanosheets. Electrochem. Commun. 2019, 109, 106609. [Google Scholar] [CrossRef]
- Mousty, C.; Walcarius, A. Electrochemically assisted deposition by local pH tuning: A versatile tool to generate ordered mesoporous silica thin films and layered double hydroxide materials. J. Solid State Electrochem. 2015, 19, 1905–1931. [Google Scholar] [CrossRef]
- Wang, S.; Ye, X.; Zhang, H.; Qian, Z.; Li, Q.; Wu, Z.; Li, S. Superhydrophobic Silane/Fluorinated Attapulgite@SiO2 Composite Coatings on Magnesium Alloy for Corrosion Protection. ChemistrySelect 2020, 5, 10329–10338. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Deepa, P.N.; Kanungo, M.; Claycomb, G.; Sherwood, P.M.A.; Collinson, M.M. Electrochemically Deposited Sol−Gel-Derived Silicate Films as a Viable Alternative in Thin-Film Design. Anal. Chem. 2003, 75, 5399–5405. [Google Scholar] [CrossRef] [PubMed]
- Collinson, M.M.; Moore, N.; Deepa, P.N.; Kanungo, M. Electrodeposition of Porous Silicate Films from Ludox Colloidal Silica. Langmuir 2003, 19, 7669–7672. [Google Scholar] [CrossRef]
- Giordano, G.; Durante, C.; Gennaro, A.; Guglielmi, M. Electrochemical deposition of silica sol–gel films on stainless steel: Preliminary analysis of key variables. J. Sol-Gel Sci. Technol. 2015, 76, 233–240. [Google Scholar] [CrossRef]
- Toledano, R.; Shacham, R.; Avnir, D.; Mandler, D. Electrochemical Co-deposition of Sol−Gel/Metal Thin Nanocomposite Films. Chem. Mater. 2008, 20, 4276–4283. [Google Scholar] [CrossRef]
- Toledano, R.; Mandler, D. Electrochemical Codeposition of Thin Gold Nanoparticles/Sol−Gel Nanocomposite Films. Chem. Mater. 2010, 22, 3943–3951. [Google Scholar] [CrossRef]
- Wu, L.-K.; Hu, J.-M.; Zhang, J.-Q. One step sol–gel electrochemistry for the fabrication of superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 14471–14475. [Google Scholar] [CrossRef]
- Farghaly, A.A.; Collinson, M.M. Electroassisted Codeposition of Sol–Gel Derived Silica Nanocomposite Directs the Fabrication of Coral-like Nanostructured Porous Gold. Langmuir 2014, 30, 5276–5286. [Google Scholar] [CrossRef]
- Gdor, E.; Mandler, D. Electrochemically assisted deposition of biodegradable polymer nanoparticles/sol–gel thin films. J. Mater. Chem. 2011, 21, 12145–12150. [Google Scholar] [CrossRef]
- Xie, J.; Hu, J.; Lin, X.; Fang, L.; Wu, F.; Liao, X.; Luo, H.; Shi, L. Robust and anti-corrosive PDMS/SiO2 superhydrophobic coatings fabricated on magnesium alloys with different-sized SiO2 nanoparticles. Appl. Surf. Sci. 2018, 457, 870–880. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Chen, R.-J.; Hu, J.-M. Superhydrophobic surface constructed on electrodeposited silica films by two-step method for corrosion protection of mild steel. Corros. Sci. 2016, 104, 336–343. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R. Liquid/solid contact mode of super-hydrophobic film in aqueous solution and its effect on corrosion resistance. Corros. Sci. 2012, 54, 77–84. [Google Scholar] [CrossRef]
- Wu, L.-K.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Superhydrophobic surface constructed on electrodeposited sol–gel silica film. Electrochem. Commun. 2013, 26, 85–88. [Google Scholar] [CrossRef]
- Wu, L.-K.; Zhang, X.-F.; Hu, J.-M. Corrosion protection of mild steel by one-step electrodeposition of superhydrophobic silica film. Corros. Sci. 2014, 85, 482–487. [Google Scholar] [CrossRef]
- Yan, W.; Ong, W.K.; Wu, Y.L.; Wijesinghe, S.L. Investigation of Using Sol-Gel Technology for Corrosion Protection Coating Systems Incorporating Colours and Inhibitors. Coatings 2019, 9, 52. [Google Scholar] [CrossRef]
- Hu, J.-M.; Liu, L.; Zhang, J.-Q.; Cao, C.-N. Effects of electrodeposition potential on the corrosion properties of bis-1,2-[triethoxysilyl] ethane films on aluminum alloy. Electrochim. Acta 2006, 51, 3944–3949. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Cao, D.-L.; Xu, L.-K.; Tang, J.-K.; Meng, R.-Q.; Li, H.-D. Corrosion resistance of a superhydrophobic dodecyltrimethoxysilane coating on magnesium alloy AZ31 fabricated by one-step electrodeposition. New J. Chem. 2021, 45, 14665–14676. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Szubert, K.; Peipmann, R.; Fritz, M.; Schmidt, U.; Bund, A.; Lota, G. Anti-corrosive properties of silane coatings deposited on anodised aluminium. Electrochim. Acta 2016, 220, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Li, J.; Zou, M.; Zheng, H. UV laser-produced copper micro-mesh with superhydrophobic-oleophilic surface for oil-water separation. J. Mater. Res. Technol. 2021, 15, 5733–5745. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Choi, W.; Tuteja, A.; Mabry, J.M.; Cohen, R.E.; McKinley, G.H. A modified Cassie–Baxter relationship to explain contact angle hysteresis and anisotropy on non-wetting textured surfaces. J. Coll. Interface Sci. 2009, 339, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Zhou, C. Anti-corrosive superhydrophobic coatings fabricated by a simple dipping method. Surf. Eng. 2021, 37, 1404–1413. [Google Scholar] [CrossRef]
- Han, J.; Liu, E.; Zhou, Y.; Zhao, S.; Yan, H.; Hu, C.; Kang, J.; Han, Q.; Su, Y. Robust superhydrophobic film on aluminum alloy prepared with TiO2/SiO2-silane composite film for efficient self-cleaning, anti-corrosion and anti-icing. Mater. Today Commun. 2023, 34, 105085. [Google Scholar] [CrossRef]
- Bai, C.; Hu, C.; Zhang, X.; Zhang, W.; Ma, B.; Li, T. A rapid two-step method for fabrication of superhydrophobic-superoleophobic nickel/copper alloy coating with self-cleaning and anticorrosion properties. Coll. Surf. A Physicochem. Eng. Asp. 2022, 651, 129635. [Google Scholar] [CrossRef]
- Zhu, M.; Yuan, R.; Wang, C.; Gao, Q.; Wang, H.; Qian, H. Fabrication and performance study of a superhydrophobic anti-scaling and anti-corrosion coating. Appl. Surf. Sci. 2023, 615, 156287. [Google Scholar] [CrossRef]
- Wang, C.-X.; Zhang, X.-F. A non-particle and fluorine-free superhydrophobic surface based on one-step electrodeposition of dodecyltrimethoxysilane on mild steel for corrosion protection. Corros. Sci. 2020, 163, 108284. [Google Scholar] [CrossRef]














| Element | Cu | Bi | Sb | As | Fe | Pb | S |
|---|---|---|---|---|---|---|---|
| Content | 99.980 | 0.001 | 0.002 | 0.002 | 0.005 | 0.005 | 0.005 |
| Substrate | Coating Material | Process Chemical Composition | Coating Methods | Contact Angle | Roughness | Thickness | Performance | Ref. |
|---|---|---|---|---|---|---|---|---|
| Al alloy | FP/SiO2 | 3.5 g SNP, 0.5 ml FAS-17, 1.2 mL KH560, 0.5 mL KH550 + FP | Two-step dipping | 152.1° | - | - | Anti-corrosion, coating adhesion | [54] |
| Carbon steel | TPU/CNTs@SiO2 | 0.2 gc-CNTs, 50 mL EtOH/H2O + TEOS + F-17 + TPU | Simple coating | 163° | - | - | Durability, chemical stability, self-cleaning and anti-fouling, anti-corrosion | [18] |
| Al alloy | TiO2-SiO2-silane | 0.5 Macetylacetone and tetrabutyl titanate + 0.5 M tetraethyl orthosilicate + 0.2 M MTES, 50 mM FAS | Anodic anodization, Chemical deposition | 165° | - | - | Stability, non-sticking, water repellency, self-cleaning, anti-icing | [55] |
| Ni/Cu | PFDS/ PNCA | 15.6 g/L CuSO4·5 H2O, 17.6 g/L NiSO4·7 H2O, 14 g/L boracic acid, 3 g/L SDS + PDFS | electrochemical codeposition | 163° | 2.241 μm | - | Mechanical and chemical stabilities, self-cleaning corrosion assessment | [56] |
| Copper alloy | PFDS/ EDTA/ ACO | Copper alloy in 3.0 mol/L NaOH, 1 mol/L EDTA, 0.243 g Tris buffer + 0.16 g DA +5 g CuSO4 + 10 g H2O2, 1 wt% PFDS | Anodised | 164.53° | - | - | Anti-scaling performance, corrosion resistance test | [57] |
| SS 306, ITO | E-SiO2 | 20 mL EtOH, 20 mL 0.1 M KNO3, 2 mL TEOS, 0.001 M HCl + DTMS | Two step electrochemical assisted deposition | >150° | >~1.0 μm | >~7.5 μm | - | [45] |
| MS | SiO2/ DTMS | 20 mL 0.2 M KNO3, 80 mL EtOH, 2 mLTEOS, 2 mL DTMS | One step electrochemical assisted deposition | >150° | 0–0.6 μm | 1–5 μm | Corrosion assessment, iron dissolution determination | [46] |
| AZ31 Mg alloys | AZ31/e-DTMS | 80 mL EtOH, 20 mL 0.2 M KNO3, 3 mL DTMS | One step electrodeposition | 158° | - | 15.6 μm | Self-cleaning performance, stability tests, corrosion behavior, hydrogen evolution test | [49] |
| MS | E-SiO2 | 50 mL EtOH, 5 mL TEOS, 50 mL 0.2 M NaNO3+ 3.0 vol.% DTMS | Two step method | 155° | 0–4 μm | 0–20 μm | Corrosion test, abrasion resistance, stability | [43] |
| ITO | E-DTMS | 2 mL DTMS, 80 mL EtOH, 20 mL 0.2 M KNO3 + 2 mL TEOS | One step sol–gel electrochemistry | >150° | 0–2.7 μm | 0–9 μm | Chemical stability, indentation tests | [39] |
| MS | SiO2 | 80/20 (v/v) EtOH/0.2 M KNO3+ 2.0 vol.% DTMS | One step electrodeposition | >150° | 0–1.1 μm | 1.3–5 μm | Stability, corrosion protection | [58] |
| Copper | E-MTES/ PFOTS | 0.2 M KNO3, 200 mL EtOH, 50 mLMTES + 2.5 g PFOTS, 35 g propanol, 0.2 g SiO2 | Two step electrochemical assisted deposition | 153° | 0.2–8.51 μm | 0.7–78.26 μm | Stability tests, self-cleaning, durability, abrasion test, chemical stability, durability | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Wu, Y.; Zheng, H. Investigation of Electrochemical Assisted Deposition of Sol-Gel Silica Films for Long-Lasting Superhydrophobicity. Materials 2023, 16, 1417. https://doi.org/10.3390/ma16041417
Zhou B, Wu Y, Zheng H. Investigation of Electrochemical Assisted Deposition of Sol-Gel Silica Films for Long-Lasting Superhydrophobicity. Materials. 2023; 16(4):1417. https://doi.org/10.3390/ma16041417
Chicago/Turabian StyleZhou, Baoming, Yongling Wu, and Hongyu Zheng. 2023. "Investigation of Electrochemical Assisted Deposition of Sol-Gel Silica Films for Long-Lasting Superhydrophobicity" Materials 16, no. 4: 1417. https://doi.org/10.3390/ma16041417
APA StyleZhou, B., Wu, Y., & Zheng, H. (2023). Investigation of Electrochemical Assisted Deposition of Sol-Gel Silica Films for Long-Lasting Superhydrophobicity. Materials, 16(4), 1417. https://doi.org/10.3390/ma16041417